Cellular senescence as a driver of cognitive decline triggered by chronic unpredictable stress
- PMID: 34095365
- PMCID: PMC8163993
- DOI: 10.1016/j.ynstr.2021.100341
Cellular senescence as a driver of cognitive decline triggered by chronic unpredictable stress
Abstract
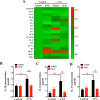
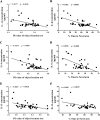
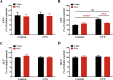
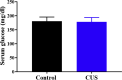
When an individual is under stress, the undesired effect on the brain often exceeds expectations. Additionally, when stress persists for a long time, it can trigger serious health problems, particularly depression. Recent studies have revealed that depressed patients have a higher rate of brain aging than healthy subjects and that depression increases dementia risk later in life. However, it remains unknown which factors are involved in brain aging triggered by chronic stress. The most critical change during brain aging is the decline in cognitive function. In addition, cellular senescence is a stable state of cell cycle arrest that occurs because of damage and/or stress and is considered a sign of aging. We used the chronic unpredictable stress (CUS) model to mimic stressful life situations and found that, compared with nonstressed control mice, CUS-treated C57BL/6 mice exhibited depression-like behaviors and cognitive decline. Additionally, the protein expression of the senescence marker p16INK4a was increased in the hippocampus, and senescence-associated β-galactosidase (SA-β-gal)-positive cells were found in the hippocampal dentate gyrus (DG) in CUS-treated mice. Furthermore, the levels of SA-β-gal or p16INK4a were strongly correlated with the severity of memory impairment in CUS-treated mice, whereas clearing senescent cells using the pharmacological senolytic cocktail dasatinib plus quercetin (D + Q) alleviated CUS-induced cognitive deficits, suggesting that targeting senescent cells may be a promising candidate approach to study chronic stress-induced cognitive decline. Our findings open new avenues for stress-related research and provide new insight into the association of chronic stress-induced cellular senescence with cognitive deficits.
Keywords: Cellular senescence; Chronic stress; Cognitive decline; Senolytics.
© 2021 The Authors.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

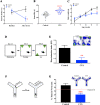

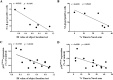
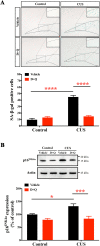
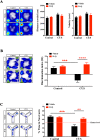
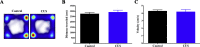
Similar articles
-
Effect of peripheral cellular senescence on brain aging and cognitive decline.Aging Cell. 2023 May;22(5):e13817. doi: 10.1111/acel.13817. Epub 2023 Mar 23. Aging Cell. 2023. PMID: 36959691 Free PMC article.
-
Targeted Reduction of Senescent Cell Burden Alleviates Focal Radiotherapy-Related Bone Loss.J Bone Miner Res. 2020 Jun;35(6):1119-1131. doi: 10.1002/jbmr.3978. Epub 2020 Mar 5. J Bone Miner Res. 2020. PMID: 32023351 Free PMC article.
-
Prophylactic senolytic treatment in aged mice reduces seizure severity and improves survival from Status Epilepticus.Aging Cell. 2024 Sep;23(9):e14239. doi: 10.1111/acel.14239. Epub 2024 Jun 20. Aging Cell. 2024. PMID: 39031751 Free PMC article.
-
Cellular Senescence in Brain Aging.Front Aging Neurosci. 2021 Feb 25;13:646924. doi: 10.3389/fnagi.2021.646924. eCollection 2021. Front Aging Neurosci. 2021. PMID: 33732142 Free PMC article. Review.
-
Aging of the cells: Insight into cellular senescence and detection Methods.Eur J Cell Biol. 2020 Aug;99(6):151108. doi: 10.1016/j.ejcb.2020.151108. Epub 2020 Jul 12. Eur J Cell Biol. 2020. PMID: 32800277 Review.
Cited by
-
The biology of aging in a social world:insights from free-ranging rhesus macaques.bioRxiv [Preprint]. 2023 Jan 29:2023.01.28.525893. doi: 10.1101/2023.01.28.525893. bioRxiv. 2023. Update in: Neurosci Biobehav Rev. 2023 Nov;154:105424. doi: 10.1016/j.neubiorev.2023.105424. PMID: 36747827 Free PMC article. Updated. Preprint.
-
Elevated Hippocampal CRMP5 Mediates Chronic Stress-Induced Cognitive Deficits by Disrupting Synaptic Plasticity, Hindering AMPAR Trafficking, and Triggering Cytokine Release.Int J Mol Sci. 2023 Mar 3;24(5):4898. doi: 10.3390/ijms24054898. Int J Mol Sci. 2023. PMID: 36902337 Free PMC article.
-
Adipose Tissue Compartments, Inflammation, and Cardiovascular Risk in the Context of Depression.Front Psychiatry. 2022 Apr 4;13:831358. doi: 10.3389/fpsyt.2022.831358. eCollection 2022. Front Psychiatry. 2022. PMID: 35444568 Free PMC article. Review.
-
Immunotherapeutic approach to reduce senescent cells and alleviate senescence-associated secretory phenotype in mice.Aging Cell. 2023 May;22(5):e13806. doi: 10.1111/acel.13806. Epub 2023 Mar 26. Aging Cell. 2023. PMID: 36967480 Free PMC article.
-
Insights into the potential benefits of triphala polyphenols toward the promotion of resilience against stress-induced depression and cognitive impairment.Curr Res Food Sci. 2023 Jun 2;6:100527. doi: 10.1016/j.crfs.2023.100527. eCollection 2023. Curr Res Food Sci. 2023. PMID: 37377497 Free PMC article. Review.
References
-
- Abelaira H.M., Réus G.Z., Quevedo J. Animal models as tools to study the pathophysiology of depression. Br. J. Psychiatr. 2013;35(Suppl. 2):S112–S120. - PubMed
-
- Antoniuk S., Bijata M., Ponimaskin E., Wlodarczyk J. Chronic unpredictable mild stress for modeling depression in rodents: meta-analysis of model reliability. Neurosci. Biobehav. Rev. 2019;99:101–116. - PubMed
-
- Bale T.L. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm. Behav. 2005;48:1–10. - PubMed
LinkOut - more resources
Full Text Sources

