Melatonin Inhibits Migration and Invasion in LPS-Stimulated and -Unstimulated Prostate Cancer Cells Through Blocking Multiple EMT-Relative Pathways
- PMID: 34079331
- PMCID: PMC8164707
- DOI: 10.2147/JIR.S305450
Melatonin Inhibits Migration and Invasion in LPS-Stimulated and -Unstimulated Prostate Cancer Cells Through Blocking Multiple EMT-Relative Pathways
Retraction in
-
Melatonin Inhibits Migration and Invasion in LPS-Stimulated and -Unstimulated Prostate Cancer Cells Through Blocking Multiple EMT-Relative Pathways [Retraction].J Inflamm Res. 2024 Feb 2;17:639-640. doi: 10.2147/JIR.S462239. eCollection 2024. J Inflamm Res. 2024. PMID: 38328559 Free PMC article.
Abstract
Purpose: Gram-negative bacteria are usually found in prostate cancer (PCa) tissues. This study aims to investigate the role of lipopolysaccharide (LPS), a glycolipid compound found in the outer membrane of gram-negative bacteria, on the migration and invasion of PCa cells, and to evaluate the protective effect of melatonin.
Materials and methods: DU145, PC-3 and LNCaP cells were incubated with LPS in the presence or absence of melatonin. Wound healing and Transwell assays were used to analyze migration and invasion of PCa cells. RT-PCR and Western blotting were used to assess the mRNA and protein levels, respectively. Co-IP was used to analyze β-catenin ubiquitination.
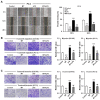
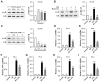
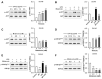
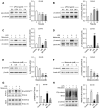
Results: Our results showed that LPS promoted migration and invasion of PCa cells. In addition, LPS stimulated inflammatory reaction and induced epithelial-mesenchymal transition (EMT) in PCa cells by activating several TLR4 downstream pathways. Specifically, LPS promoted NF-κB/IL-6/STAT3 signal transduction. In addition, LPS upregulated phosphorylation levels of cytoplasmic AKTSer473 and GSK-3βSer9. Moreover, LPS induced phosphorylation of GSK-3βSer9 in the "disruption complex", and then inhibited phosphorylation and ubiquitination of cytoplasmic β-catenin, leading to β-catenin nuclear translocation. Interestingly, melatonin inhibited invasion and migration not only in LPS-stimulated but also in LPS-unstimulated PCa cells. Melatonin suppressed PCa cells migration and invasion by blocking EMT mediated by IL-6/STAT3, AKT/GSK-3β and β-catenin pathways.
Conclusion: This study provides evidence that melatonin inhibits migration and invasion through blocking multiple TLR4 downstream EMT-associated pathways both in LPS-stimulated and -unstimulated PCa cells. Our results provide new insights into the role of bacterial infection in PCa metastasis and a potential therapeutic agent.
Keywords: EMT; lipopolysaccharide; melatonin; prostate cancer; β-catenin.
© 2021 Tian et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Melatonin prevents LPS-induced epithelial-mesenchymal transition in human alveolar epithelial cells via the GSK-3β/Nrf2 pathway.Biomed Pharmacother. 2020 Dec;132:110827. doi: 10.1016/j.biopha.2020.110827. Epub 2020 Oct 13. Biomed Pharmacother. 2020. PMID: 33065391
-
Calcitriol inhibits lipopolysaccharide-induced proliferation, migration and invasion of prostate cancer cells through suppressing STAT3 signal activation.Int Immunopharmacol. 2020 Feb 28;82:106346. doi: 10.1016/j.intimp.2020.106346. Online ahead of print. Int Immunopharmacol. 2020. PMID: 32120344
-
β-ionone Inhibits Epithelial-Mesenchymal Transition (EMT) in Prostate Cancer Cells by Negatively Regulating the Wnt/β-Catenin Pathway.Front Biosci (Landmark Ed). 2022 Dec 28;27(12):335. doi: 10.31083/j.fbl2712335. Front Biosci (Landmark Ed). 2022. PMID: 36624947
-
Role of Wnt/beta-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1alpha.Int J Urol. 2007 Nov;14(11):1034-9. doi: 10.1111/j.1442-2042.2007.01866.x. Int J Urol. 2007. PMID: 17956532
-
Calcitriol inhibits migration and invasion of renal cell carcinoma cells by suppressing Smad2/3-, STAT3- and β-catenin-mediated epithelial-mesenchymal transition.Cancer Sci. 2020 Jan;111(1):59-71. doi: 10.1111/cas.14237. Cancer Sci. 2020. PMID: 31729097 Free PMC article.
Cited by
-
Use of Melatonin in Cancer Treatment: Where Are We?Int J Mol Sci. 2022 Mar 29;23(7):3779. doi: 10.3390/ijms23073779. Int J Mol Sci. 2022. PMID: 35409137 Free PMC article. Review.
-
Prostate Microbiota and Prostate Cancer: A New Trend in Treatment.Front Oncol. 2021 Dec 10;11:805459. doi: 10.3389/fonc.2021.805459. eCollection 2021. Front Oncol. 2021. PMID: 34956913 Free PMC article. Review.
-
Bacterial Lipopolysaccharide Augmented Malignant Transformation and Promoted the Stemness in Prostate Cancer Epithelial Cells.J Inflamm Res. 2021 Nov 9;14:5849-5862. doi: 10.2147/JIR.S332943. eCollection 2021. J Inflamm Res. 2021. PMID: 34785925 Free PMC article.
-
6-Gingerol suppresses cell viability, migration and invasion via inhibiting EMT, and inducing autophagy and ferroptosis in LPS-stimulated and LPS-unstimulated prostate cancer cells.Oncol Lett. 2022 Jun;23(6):187. doi: 10.3892/ol.2022.13307. Epub 2022 Apr 26. Oncol Lett. 2022. PMID: 35527779 Free PMC article.
-
Ubiquitination Process Mediates Prostate Cancer Development and Metastasis through Multiple Mechanisms.Cell Biochem Biophys. 2024 Mar;82(1):77-90. doi: 10.1007/s12013-023-01156-x. Epub 2023 Oct 17. Cell Biochem Biophys. 2024. PMID: 37847340 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

