Transition state analogue of MTAP extends lifespan of APCMin/+ mice
- PMID: 33893330
- PMCID: PMC8065027
- DOI: 10.1038/s41598-021-87734-6
Transition state analogue of MTAP extends lifespan of APCMin/+ mice
Abstract
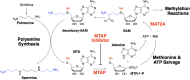
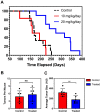
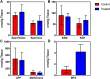
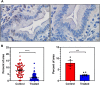
A mouse model of human Familial Adenomatous Polyposis responds favorably to pharmacological inhibition of 5'-methylthioadenosine phosphorylase (MTAP). Methylthio-DADMe-Immucillin-A (MTDIA) is an orally available, transition state analogue inhibitor of MTAP. 5'-Methylthioadenosine (MTA), the substrate for MTAP, is formed in polyamine synthesis and is recycled by MTAP to S-adenosyl-L-methionine (SAM) via salvage pathways. MTDIA treatment causes accumulation of MTA, which inhibits growth of human head and neck (FaDu) and lung (H359, A549) cancers in immunocompromised mouse models. We investigated the efficacy of oral MTDIA as an anti-cancer therapeutic for intestinal adenomas in immunocompetent APCMin/+ mice, a murine model of human Familial Adenomatous Polyposis. Tumors in APCMin/+ mice were decreased in size by MTDIA treatment, resulting in markedly improved anemia and doubling of mouse lifespan. Metabolomic analysis of treated mice showed no changes in polyamine, methionine, SAM or ATP levels when compared with control mice but indicated an increase in MTA, the MTAP substrate. Generation of an MTDIA-resistant cell line in culture showed a four-fold amplification of the methionine adenosyl transferase (MAT2A) locus and expression of this enzyme. MAT2A is downstream of MTAP action and catalyzes synthesis of the SAM necessary for methylation reactions. Immunohistochemical analysis of treated mouse intestinal tissue demonstrated a decrease in symmetric dimethylarginine, a PRMT5-catalyzed modification. The anti-cancer effects of MTDIA indicate that increased cellular MTA inhibits PRMT5-mediated methylations resulting in attenuated tumor growth. Oral dosing of MTDIA as monotherapy has potential for delaying the onset and progression of colorectal cancers in Familial Adenomatous Polyposis (FAP) as well as residual duodenal tumors in FAP patients following colectomy. MTDIA causes a physiologic inactivation of MTAP and may also have efficacy in combination with inhibitors of MAT2A or PRMT5, known synthetic-lethal interactions in MTAP-/- cancer cell lines.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Combined inhibition of MTAP and MAT2a mimics synthetic lethality in tumor models via PRMT5 inhibition.J Biol Chem. 2024 Jan;300(1):105492. doi: 10.1016/j.jbc.2023.105492. Epub 2023 Nov 23. J Biol Chem. 2024. PMID: 38000655 Free PMC article.
-
Growth and metastases of human lung cancer are inhibited in mouse xenografts by a transition state analogue of 5'-methylthioadenosine phosphorylase.J Biol Chem. 2011 Feb 11;286(6):4902-11. doi: 10.1074/jbc.M110.198374. Epub 2010 Dec 6. J Biol Chem. 2011. PMID: 21135097 Free PMC article.
-
Phosphoinositide and redox dysregulation by the anticancer methylthioadenosine phosphorylase transition state inhibitor.Biochim Biophys Acta Mol Cell Biol Lipids. 2023 Sep;1868(9):159346. doi: 10.1016/j.bbalip.2023.159346. Epub 2023 Jun 8. Biochim Biophys Acta Mol Cell Biol Lipids. 2023. PMID: 37301365
-
The potential and challenges of targeting MTAP-negative cancers beyond synthetic lethality.Front Oncol. 2023 Sep 19;13:1264785. doi: 10.3389/fonc.2023.1264785. eCollection 2023. Front Oncol. 2023. PMID: 37795443 Free PMC article. Review.
-
Targeting tumors that lack methylthioadenosine phosphorylase (MTAP) activity: current strategies.Cancer Biol Ther. 2011 Apr 1;11(7):627-32. doi: 10.4161/cbt.11.7.14948. Epub 2011 Apr 1. Cancer Biol Ther. 2011. PMID: 21301207 Free PMC article. Review.
Cited by
-
Adenine crosses the biomarker bridge: from 'omics to treatment in diabetic kidney disease.J Clin Invest. 2023 Oct 16;133(20):e174015. doi: 10.1172/JCI174015. J Clin Invest. 2023. PMID: 37843281 Free PMC article.
-
Endogenous adenine mediates kidney injury in diabetic models and predicts diabetic kidney disease in patients.J Clin Invest. 2023 Oct 16;133(20):e170341. doi: 10.1172/JCI170341. J Clin Invest. 2023. PMID: 37616058 Free PMC article.
-
Metabolic Heterogeneity, Plasticity, and Adaptation to "Glutamine Addiction" in Cancer Cells: The Role of Glutaminase and the GTωA [Glutamine Transaminase-ω-Amidase (Glutaminase II)] Pathway.Biology (Basel). 2023 Aug 14;12(8):1131. doi: 10.3390/biology12081131. Biology (Basel). 2023. PMID: 37627015 Free PMC article. Review.
References
-
- Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl. J. Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. - DOI - PMC - PubMed
-
- Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, Sargeant L, Krapcho K, Wolff E, Burt R, Hughes JP, Warrington J, McPherson J, Wasmuth J, Le Paslier D, Abderrahim H, Cohen D, Leppert M, White R. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

