Immune checkpoint inhibitor-associated myocarditis: manifestations and mechanisms
- PMID: 33645548
- PMCID: PMC7919710
- DOI: 10.1172/JCI145186
Immune checkpoint inhibitor-associated myocarditis: manifestations and mechanisms
Abstract
Immune checkpoint inhibitors (ICIs) have transformed the treatment of various cancers, including malignancies once considered untreatable. These agents, however, are associated with inflammation and tissue damage in multiple organs. Myocarditis has emerged as a serious ICI-associated toxicity, because, while seemingly infrequent, it is often fulminant and lethal. The underlying basis of ICI-associated myocarditis is not completely understood. While the importance of T cells is clear, the inciting antigens, why they are recognized, and the mechanisms leading to cardiac cell injury remain poorly characterized. These issues underscore the need for basic and clinical studies to define pathogenesis, identify predictive biomarkers, improve diagnostic strategies, and develop effective treatments. An improved understanding of ICI-associated myocarditis will provide insights into the equilibrium between the immune and cardiovascular systems.
Conflict of interest statement
Figures
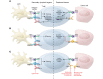
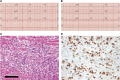
Similar articles
-
Cardiotoxicity of Immune Checkpoint Inhibitors.Curr Oncol Rep. 2021 May 3;23(7):79. doi: 10.1007/s11912-021-01070-6. Curr Oncol Rep. 2021. PMID: 33937956 Free PMC article. Review.
-
[Research Progress of Immune Checkpoint Inhibitor-associated Myocarditis].Zhongguo Fei Ai Za Zhi. 2021 Sep 20;24(9):668-672. doi: 10.3779/j.issn.1009-3419.2021.102.27. Epub 2021 Sep 15. Zhongguo Fei Ai Za Zhi. 2021. PMID: 34521189 Free PMC article. Chinese.
-
Immune-checkpoint inhibitor-mediated myocarditis: CTLA4, PD1 and LAG3 in the heart.Nat Rev Cancer. 2024 Aug;24(8):540-553. doi: 10.1038/s41568-024-00715-5. Epub 2024 Jul 9. Nat Rev Cancer. 2024. PMID: 38982146 Review.
-
Clinical Strategy for the Diagnosis and Treatment of Immune Checkpoint Inhibitor-Associated Myocarditis: A Narrative Review.JAMA Cardiol. 2021 Nov 1;6(11):1329-1337. doi: 10.1001/jamacardio.2021.2241. JAMA Cardiol. 2021. PMID: 34232253 Review.
-
Single-cell RNA sequencing reveals the altered innate immunity in immune checkpoint inhibitor-related myocarditis.Immunology. 2024 Jun;172(2):235-251. doi: 10.1111/imm.13770. Epub 2024 Feb 29. Immunology. 2024. PMID: 38425094
Cited by
-
Clinical characteristics and prognosis of pancreatitis associated with immune checkpoint inhibitors.Clin Transl Oncol. 2025 Jan;27(1):333-339. doi: 10.1007/s12094-024-03573-7. Epub 2024 Jul 12. Clin Transl Oncol. 2025. PMID: 38995514
-
Adverse events of immune checkpoint inhibitors for patients with digestive system cancers: A systematic review and meta-analysis.Front Immunol. 2022 Oct 21;13:1013186. doi: 10.3389/fimmu.2022.1013186. eCollection 2022. Front Immunol. 2022. PMID: 36341450 Free PMC article.
-
Cardiac fibrosis in oncologic therapies.Curr Opin Physiol. 2022 Oct;29:100575. doi: 10.1016/j.cophys.2022.100575. Epub 2022 Aug 8. Curr Opin Physiol. 2022. PMID: 36187050 Free PMC article.
-
LAG3 Regulates T Cell Activation and Plaque Infiltration in Atherosclerotic Mice.JACC CardioOncol. 2022 Dec 20;4(5):635-645. doi: 10.1016/j.jaccao.2022.09.005. eCollection 2022 Dec. JACC CardioOncol. 2022. PMID: 36636446 Free PMC article.
-
Overexpression of TPM4 is associated with worse prognosis and immune infiltration in patients with glioma.BMC Neurol. 2023 Jan 13;23(1):17. doi: 10.1186/s12883-023-03058-0. BMC Neurol. 2023. PMID: 36639743 Free PMC article.
References
-
- Wei SC, et al. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–1086. doi: 10.1158/2159-8290.CD-18-0367. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

