Characteristics of Epigenetic Clocks Across Blood and Brain Tissue in Older Women and Men
- PMID: 33488342
- PMCID: PMC7817909
- DOI: 10.3389/fnins.2020.555307
Characteristics of Epigenetic Clocks Across Blood and Brain Tissue in Older Women and Men
Abstract
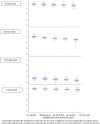
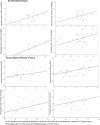
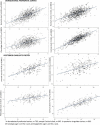
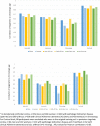
Epigenetic clocks are among the most promising biomarkers of aging. It is particularly important to establish biomarkers of brain aging to better understand neurodegenerative diseases. To advance application of epigenetic clocks-which were largely created with DNA methylation levels in blood samples-for use in brain, we need clearer evaluation of epigenetic clock behavior in brain, including direct comparisons of brain specimens with blood, a more accessible tissue for research. We leveraged data from the Religious Orders Study and Rush Memory and Aging Project to examine three established epigenetic clocks (Horvath, Hannum, PhenoAge clocks) and a newer clock, trained in cortical tissue. We calculated each clock in three different specimens: (1) antemortem CD4+ cells derived from blood (n = 41); (2) postmortem dorsolateral prefrontal cortex (DLPFC, n = 730); and (3) postmortem posterior cingulate cortex (PCC, n = 186), among older women and men, age 66-108 years at death. Across all clocks, epigenetic age calculated from blood and brain specimens was generally lower than chronologic age, although differences were smallest for the Cortical clock when calculated in the brain specimens. Nonetheless, we found that Pearson correlations of epigenetic to chronologic ages in brain specimens were generally reasonable for all clocks; correlations for the Horvath, Hannum, and PhenoAge clocks largely ranged from 0.5 to 0.7 (all p < 0.0001). The Cortical clock outperformed the other clocks, reaching a correlation of 0.83 in the DLFPC (p < 0.0001) for epigenetic vs. chronologic age. Nonetheless, epigenetic age was quite modestly correlated across blood and DLPFC in 41 participants with paired samples [Pearson r from 0.21 (p = 0.2) to 0.32 (p = 0.05)], indicating that broader research in neurodegeneration may benefit from clocks using CpG sites better conserved across blood and brain. Finally, in analyses stratified by sex, by pathologic diagnosis of Alzheimer disease, and by clinical diagnosis of Alzheimer dementia, correlations of epigenetic to chronologic age remained consistently high across all groups. Future research in brain aging will benefit from epigenetic clocks constructed in brain specimens, including exploration of any advantages of focusing on CpG sites conserved across brain and other tissue types.
Keywords: aging; biomarkers; dementia; epigenetics; neurology.
Copyright © 2021 Grodstein, Lemos, Yu, Iatrou, De Jager and Bennett.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The association of epigenetic clocks in brain tissue with brain pathologies and common aging phenotypes.Neurobiol Dis. 2021 Sep;157:105428. doi: 10.1016/j.nbd.2021.105428. Epub 2021 Jun 19. Neurobiol Dis. 2021. PMID: 34153464 Free PMC article.
-
Advancing understanding of maternal age: correlating epigenetic clocks in blood and myometrium.Epigenetics Commun. 2022;2:3. doi: 10.1186/s43682-022-00010-0. Epub 2022 May 23. Epigenetics Commun. 2022. PMID: 36052275 Free PMC article.
-
Recalibrating the epigenetic clock: implications for assessing biological age in the human cortex.Brain. 2020 Dec 1;143(12):3763-3775. doi: 10.1093/brain/awaa334. Brain. 2020. PMID: 33300551 Free PMC article.
-
A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration.Ageing Res Rev. 2021 Aug;69:101348. doi: 10.1016/j.arr.2021.101348. Epub 2021 Apr 28. Ageing Res Rev. 2021. PMID: 33930583 Review.
-
Epigenetic clocks provide clues to the mystery of uterine ageing.Hum Reprod Update. 2023 May 2;29(3):259-271. doi: 10.1093/humupd/dmac042. Hum Reprod Update. 2023. PMID: 36515535 Review.
Cited by
-
The Role of Epigenetics in Neuroinflammatory-Driven Diseases.Int J Mol Sci. 2022 Dec 2;23(23):15218. doi: 10.3390/ijms232315218. Int J Mol Sci. 2022. PMID: 36499544 Free PMC article. Review.
-
Emerging Roles of T Helper Cells in Non-Infectious Neuroinflammation: Savior or Sinner.Front Immunol. 2022 Jun 30;13:872167. doi: 10.3389/fimmu.2022.872167. eCollection 2022. Front Immunol. 2022. PMID: 35844577 Free PMC article. Review.
-
Turning Back the Clock: A Retrospective Single-Blind Study on Brain Age Change in Response to Nutraceuticals Supplementation vs. Lifestyle Modifications.Brain Sci. 2023 Mar 21;13(3):520. doi: 10.3390/brainsci13030520. Brain Sci. 2023. PMID: 36979330 Free PMC article.
-
Accelerated Epigenetic Aging in Peripheral Blood does not Predict Dementia Risk.Curr Alzheimer Res. 2021;18(5):443-451. doi: 10.2174/1567205018666210823100721. Curr Alzheimer Res. 2021. PMID: 34429046 Free PMC article.
-
Biological aging processes underlying cognitive decline and neurodegenerative disease.J Clin Invest. 2022 May 16;132(10):e158453. doi: 10.1172/JCI158453. J Clin Invest. 2022. PMID: 35575089 Free PMC article. Review.
References
-
- Alzheimer’s Association (2019). 2019 Alzheimer’s disease facts and figures. Alzheimers Dement 15 321–387. 10.1016/j.jalz.2019.01.010 - DOI
-
- Bennett D. A., Schneider J. A., Aggarwal N. T., Arvanitakis Z., Shah R. C., Kelly J. F., et al. (2006). Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology 27 169–176. 10.1159/000096129 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

