Highly redundant neuropeptide volume co-transmission underlying episodic activation of the GnRH neuron dendron
- PMID: 33464205
- PMCID: PMC7847305
- DOI: 10.7554/eLife.62455
Highly redundant neuropeptide volume co-transmission underlying episodic activation of the GnRH neuron dendron
Abstract
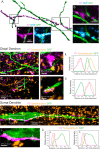
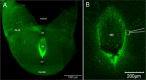
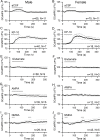
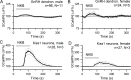
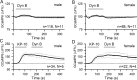
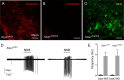
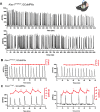
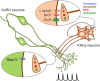
The necessity and functional significance of neurotransmitter co-transmission remains unclear. The glutamatergic 'KNDy' neurons co-express kisspeptin, neurokinin B (NKB), and dynorphin and exhibit a highly stereotyped synchronized behavior that reads out to the gonadotropin-releasing hormone (GnRH) neuron dendrons to drive episodic hormone secretion. Using expansion microscopy, we show that KNDy neurons make abundant close, non-synaptic appositions with the GnRH neuron dendron. Electrophysiology and confocal GCaMP6 imaging demonstrated that, despite all three neuropeptides being released from KNDy terminals, only kisspeptin was able to activate the GnRH neuron dendron. Mice with a selective deletion of kisspeptin from KNDy neurons failed to exhibit pulsatile hormone secretion but maintained synchronized episodic KNDy neuron behavior that is thought to depend on recurrent NKB and dynorphin transmission. This indicates that KNDy neurons drive episodic hormone secretion through highly redundant neuropeptide co-transmission orchestrated by differential post-synaptic neuropeptide receptor expression at the GnRH neuron dendron and KNDy neuron.
Keywords: Dynorphin; GCaMP; GnRH; NKB; kisspeptin; mouse; neuroscience; pulse generator.
© 2021, Liu et al.
Conflict of interest statement
XL, SY, HM, MH, SH, IC, RP, AH No competing interests declared
Figures

Similar articles
-
Indirect Suppression of Pulsatile LH Secretion by CRH Neurons in the Female Mouse.Endocrinology. 2021 Mar 1;162(3):bqaa237. doi: 10.1210/endocr/bqaa237. Endocrinology. 2021. PMID: 33543235
-
Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes.Endocrinology. 2013 Nov;154(11):4259-69. doi: 10.1210/en.2013-1331. Epub 2013 Aug 19. Endocrinology. 2013. PMID: 23959940 Free PMC article.
-
Local administration of Neurokinin B in the arcuate nucleus accelerates the neural activity of the GnRH pulse generator in goats.J Reprod Dev. 2021 Dec 14;67(6):352-358. doi: 10.1262/jrd.2021-055. Epub 2021 Oct 8. J Reprod Dev. 2021. PMID: 34629331 Free PMC article.
-
Neuroendocrine control of gonadotropin-releasing hormone: Pulsatile and surge modes of secretion.J Neuroendocrinol. 2022 May;34(5):e13094. doi: 10.1111/jne.13094. Epub 2022 Feb 2. J Neuroendocrinol. 2022. PMID: 35107859 Free PMC article. Review.
-
Importance of neuroanatomical data from domestic animals to the development and testing of the KNDy hypothesis for GnRH pulse generation.Domest Anim Endocrinol. 2020 Oct;73:106441. doi: 10.1016/j.domaniend.2020.106441. Epub 2020 Jan 24. Domest Anim Endocrinol. 2020. PMID: 32113801 Free PMC article. Review.
Cited by
-
Role of Posterodorsal Medial Amygdala Urocortin-3 in Pubertal Timing in Female Mice.Front Endocrinol (Lausanne). 2022 May 17;13:893029. doi: 10.3389/fendo.2022.893029. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35655799 Free PMC article.
-
A Modified Ultra-Sensitive ELISA for Measurement of LH in Mice.Endocrinology. 2022 Sep 1;163(9):bqac109. doi: 10.1210/endocr/bqac109. Endocrinology. 2022. PMID: 35869782 Free PMC article.
-
Transcriptome profiling of kisspeptin neurons from the mouse arcuate nucleus reveals new mechanisms in estrogenic control of fertility.Proc Natl Acad Sci U S A. 2022 Jul 5;119(27):e2113749119. doi: 10.1073/pnas.2113749119. Epub 2022 Jun 28. Proc Natl Acad Sci U S A. 2022. PMID: 35763574 Free PMC article.
-
Kisspeptin signaling in astrocytes modulates the reproductive axis.J Clin Invest. 2024 Jun 11;134(15):e172908. doi: 10.1172/JCI172908. J Clin Invest. 2024. PMID: 38861336 Free PMC article.
-
Modelling KNDy neurons and gonadotropin-releasing hormone pulse generation.Curr Opin Endocr Metab Res. 2022 Dec;27:100407. doi: 10.1016/j.coemr.2022.100407. Curr Opin Endocr Metab Res. 2022. PMID: 36632147 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

