Increased FOXM1 Expression by Cisplatin Inhibits Paclitaxel-Related Apoptosis in Cisplatin-Resistant Human Oral Squamous Cell Carcinoma (OSCC) Cell Lines
- PMID: 33255409
- PMCID: PMC7727786
- DOI: 10.3390/ijms21238897
Increased FOXM1 Expression by Cisplatin Inhibits Paclitaxel-Related Apoptosis in Cisplatin-Resistant Human Oral Squamous Cell Carcinoma (OSCC) Cell Lines
Abstract
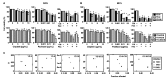
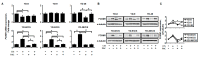
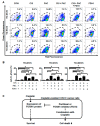
Cisplatin and paclitaxel are commonly used to treat oral cancer, but their use is often limited because of acquired drug resistance. Here, we tested the effects of combined cisplatin and paclitaxel on three parental (YD-8, YD-9, and YD-38) and three cisplatin-resistant (YD-8/CIS, YD-9/CIS, and YD-38/CIS) oral squamous cell carcinoma (OSCC) cell lines using cell proliferation assays and combination index analysis. We detected forkhead box protein M1 (FOXM1) mRNA and protein expression via real-time qPCR and Western blot assays. Cell death of the cisplatin-resistant cell lines in response to these drugs with or without a FOXM1 inhibitor (forkhead domain inhibitory compound 6) was then measured by propidium iodide staining and TdT dUTP nick end labeling (TUNEL) assays. In all six OSCC cell lines, cell growth was more inhibited by paclitaxel alone than combination therapy. Cisplatin-induced overexpression of FOXM1 showed the same trend only in cisplatin-resistant cell lines, indicating that it was associated with inhibition of paclitaxel-related apoptosis. In summary, these results suggest that, in three cisplatin-resistant cell lines, the combination of cisplatin and paclitaxel had an antagonistic effect, likely because cisplatin blocks paclitaxel-induced apoptosis. Cisplatin-induced FOXM1 overexpression may explain the failure of this combination.
Keywords: FOXM1; OSCC; apoptosis; cisplatin; paclitaxel.
Conflict of interest statement
The authors declare no conflict of interest related to this work.
Figures

Similar articles
-
DDX3 modulates cisplatin resistance in OSCC through ALKBH5-mediated m6A-demethylation of FOXM1 and NANOG.Apoptosis. 2020 Apr;25(3-4):233-246. doi: 10.1007/s10495-020-01591-8. Apoptosis. 2020. PMID: 31974865
-
Targeting FOXM1 Improves Cytotoxicity of Paclitaxel and Cisplatinum in Platinum-Resistant Ovarian Cancer.Int J Gynecol Cancer. 2017 Oct;27(8):1602-1609. doi: 10.1097/IGC.0000000000001063. Int J Gynecol Cancer. 2017. PMID: 28692634
-
FOXM1-Mediated Regulation of Reactive Oxygen Species and Radioresistance in Oral Squamous Cell Carcinoma Cells.Lab Invest. 2023 May;103(5):100060. doi: 10.1016/j.labinv.2022.100060. Epub 2023 Jan 10. Lab Invest. 2023. PMID: 36801643
-
Targeting Foxm1 Improves Cytotoxicity of Paclitaxel and Cisplatinum in Platinum-Resistant Ovarian Cancer.Int J Gynecol Cancer. 2017 Jun;27(5):887-894. doi: 10.1097/IGC.0000000000000969. Int J Gynecol Cancer. 2017. PMID: 28498253
-
The Molecular Basis and Therapeutic Aspects of Cisplatin Resistance in Oral Squamous Cell Carcinoma.Front Oncol. 2021 Oct 22;11:761379. doi: 10.3389/fonc.2021.761379. eCollection 2021. Front Oncol. 2021. PMID: 34746001 Free PMC article. Review.
Cited by
-
Transcription factor FoxM1 promotes cyst growth in PKD1 mutant ADPKD.Hum Mol Genet. 2023 Mar 20;32(7):1114-1126. doi: 10.1093/hmg/ddac273. Hum Mol Genet. 2023. PMID: 36322156 Free PMC article.
-
Oral Cavity Squamous Cell Carcinoma: An Update of the Pharmacological Treatment.Biomedicines. 2023 Apr 7;11(4):1112. doi: 10.3390/biomedicines11041112. Biomedicines. 2023. PMID: 37189730 Free PMC article. Review.
-
Cisplatin Plus Cetuximab Inhibits Cisplatin-Resistant Human Oral Squamous Cell Carcinoma Cell Migration and Proliferation but Does Not Enhance Apoptosis.Int J Mol Sci. 2021 Jul 29;22(15):8167. doi: 10.3390/ijms22158167. Int J Mol Sci. 2021. PMID: 34360933 Free PMC article.
-
Gastrodin overcomes chemoresistance via inhibiting Skp2-mediated glycolysis.Cell Death Discov. 2023 Oct 2;9(1):364. doi: 10.1038/s41420-023-01648-y. Cell Death Discov. 2023. PMID: 37779163 Free PMC article.
-
Assessing Gene Expression Related to Cisplatin Resistance in Human Oral Squamous Cell Carcinoma Cell Lines.Pharmaceuticals (Basel). 2022 Jun 3;15(6):704. doi: 10.3390/ph15060704. Pharmaceuticals (Basel). 2022. PMID: 35745623 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

