MiR-26a-5p inhibits GSK3β expression and promotes cardiac hypertrophy in vitro
- PMID: 33240671
- PMCID: PMC7678492
- DOI: 10.7717/peerj.10371
MiR-26a-5p inhibits GSK3β expression and promotes cardiac hypertrophy in vitro
Abstract
Background: The role of miR-26a-5p expression in cardiac hypertrophy remains unclear. Herein, the effect of miR-26a-5p on cardiac hypertrophy was investigated using phenylephrine (PE)-induced cardiac hypertrophy in vitro and in a rat model of hypertension-induced hypertrophy in vivo.
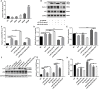
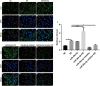
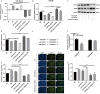
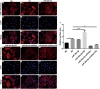
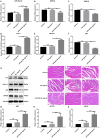
Methods: The PE-induced cardiac hypertrophy models in vitro and vivo were established. To investigate the effect of miR-26a-5p activation on autophagy, the protein expression of autophagosome marker (LC3) and p62 was detected by western blot analysis. To explore the effect of miR-26a-5p activation on cardiac hypertrophy, the relative mRNA expression of cardiac hypertrophy related mark GSK3β was detected by qRT-PCR in vitro and vivo. In addition, immunofluorescence staining was used to detect cardiac hypertrophy related mark α-actinin. The cell surface area was measured by immunofluorescence staining. The direct target relationship between miR-26a-5p and GSK3β was confirmed by dual luciferase report.
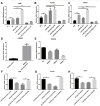
Results: MiR-26a-5p was highly expressed in PE-induced cardiac hypertrophy. MiR-26a-5p promoted LC3II and decreased p62 expression in PE-induced cardiac hypertrophy in the presence or absence of lysosomal inhibitor. Furthermore, miR-26a-5p significantly inhibited GSK3β expression in vitro and in vivo. Dual luciferase report results confirmed that miR-26a-5p could directly target GSK3β. GSK3β overexpression significantly reversed the expression of cardiac hypertrophy-related markers including ANP, ACTA1 and MYH7. Immunofluorescence staining results demonstrated that miR-26a-5p promoted cardiac hypertrophy related protein α-actinin expression, and increased cell surface area in vitro and in vivo.
Conclusion: Our study revealed that miR-26a-5p promotes myocardial cell autophagy activation and cardiac hypertrophy by regulating GSK3β, which needs further research.
Keywords: Autophagy; Cardiac hypertrophy; GSK3β; LC3; MiR-26a-5p; α-actinin.
©2020 Tang et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures
Similar articles
-
The molecular mechanism of MiR-26a-5p regulates autophagy and activates NLRP3 inflammasome to mediate cardiomyocyte hypertrophy.BMC Cardiovasc Disord. 2024 Jan 3;24(1):18. doi: 10.1186/s12872-023-03695-w. BMC Cardiovasc Disord. 2024. PMID: 38172711 Free PMC article.
-
Qiliqiangxin Attenuates Phenylephrine-Induced Cardiac Hypertrophy through Downregulation of MiR-199a-5p.Cell Physiol Biochem. 2016;38(5):1743-51. doi: 10.1159/000443113. Epub 2016 May 9. Cell Physiol Biochem. 2016. PMID: 27161004
-
MiR-26a-5p alleviates cardiac hypertrophy and dysfunction via targeting ADAM17.Cell Biol Int. 2021 Nov;45(11):2357-2367. doi: 10.1002/cbin.11685. Epub 2021 Aug 23. Cell Biol Int. 2021. PMID: 34370360
-
Melatonin Plays a Protective Role by Regulating miR-26a-5p-NRSF and JAK2-STAT3 Pathway to Improve Autophagy, Inflammation and Oxidative Stress of Cerebral Ischemia-Reperfusion Injury.Drug Des Devel Ther. 2020 Aug 6;14:3177-3188. doi: 10.2147/DDDT.S262121. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32821085 Free PMC article.
-
MiR-100-5p regulates cardiac hypertrophy through activation of autophagy by targeting mTOR.Hum Cell. 2021 Sep;34(5):1388-1397. doi: 10.1007/s13577-021-00566-4. Epub 2021 Jun 17. Hum Cell. 2021. PMID: 34138410
Cited by
-
RNA binding Motif protein-38 regulates myocardial hypertrophy in LXR-α-dependent lipogenesis pathway.Bioengineered. 2021 Dec;12(2):9655-9667. doi: 10.1080/21655979.2021.1977552. Bioengineered. 2021. PMID: 34854353 Free PMC article.
-
Long-term culture of patient-derived cardiac organoids recapitulated Duchenne muscular dystrophy cardiomyopathy and disease progression.Front Cell Dev Biol. 2022 Aug 11;10:878311. doi: 10.3389/fcell.2022.878311. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36035984 Free PMC article.
-
The molecular mechanism of MiR-26a-5p regulates autophagy and activates NLRP3 inflammasome to mediate cardiomyocyte hypertrophy.BMC Cardiovasc Disord. 2024 Jan 3;24(1):18. doi: 10.1186/s12872-023-03695-w. BMC Cardiovasc Disord. 2024. PMID: 38172711 Free PMC article.
-
Extracellular vesicles and Duchenne muscular dystrophy pathology: Modulators of disease progression.Front Physiol. 2023 Feb 14;14:1130063. doi: 10.3389/fphys.2023.1130063. eCollection 2023. Front Physiol. 2023. PMID: 36891137 Free PMC article. Review.
-
MicroRNA-194 inhibits isoproterenol-induced chronic cardiac hypertrophy via targeting CnA/NFATc2 signaling in H9c2 cells.Ann Transl Med. 2022 Jul;10(14):780. doi: 10.21037/atm-22-1894. Ann Transl Med. 2022. PMID: 35965805 Free PMC article.
References
-
- Chen Q, Zhang L, Chen S, Huang Y, Li K, Yu X, Wu H, Tian X, Zhang C, Tang C, Du J, Jin H. Downregulated endogenous sulfur dioxide/aspartate aminotransferase pathway is involved in angiotensin II-stimulated cardiomyocyte autophagy and myocardial hypertrophy in mice. International Journal of Cardiology. 2016;225:392–401. doi: 10.1016/j.ijcard.2016.09.111. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

