SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19
- PMID: 33113295
- PMCID: PMC7646625
- DOI: 10.1056/NEJMoa2029849
SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (Covid-19), which is most frequently mild yet can be severe and life-threatening. Virus-neutralizing monoclonal antibodies are predicted to reduce viral load, ameliorate symptoms, and prevent hospitalization.
Methods: In this ongoing phase 2 trial involving outpatients with recently diagnosed mild or moderate Covid-19, we randomly assigned 452 patients to receive a single intravenous infusion of neutralizing antibody LY-CoV555 in one of three doses (700 mg, 2800 mg, or 7000 mg) or placebo and evaluated the quantitative virologic end points and clinical outcomes. The primary outcome was the change from baseline in the viral load at day 11. The results of a preplanned interim analysis as of September 5, 2020, are reported here.
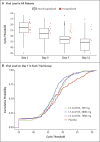
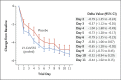
Results: At the time of the interim analysis, the observed mean decrease from baseline in the log viral load for the entire population was -3.81, for an elimination of more than 99.97% of viral RNA. For patients who received the 2800-mg dose of LY-CoV555, the difference from placebo in the decrease from baseline was -0.53 (95% confidence interval [CI], -0.98 to -0.08; P = 0.02), for a viral load that was lower by a factor of 3.4. Smaller differences from placebo in the change from baseline were observed among the patients who received the 700-mg dose (-0.20; 95% CI, -0.66 to 0.25; P = 0.38) or the 7000-mg dose (0.09; 95% CI, -0.37 to 0.55; P = 0.70). On days 2 to 6, the patients who received LY-CoV555 had a slightly lower severity of symptoms than those who received placebo. The percentage of patients who had a Covid-19-related hospitalization or visit to an emergency department was 1.6% in the LY-CoV555 group and 6.3% in the placebo group.
Conclusions: In this interim analysis of a phase 2 trial, one of three doses of neutralizing antibody LY-CoV555 appeared to accelerate the natural decline in viral load over time, whereas the other doses had not by day 11. (Funded by Eli Lilly; BLAZE-1 ClinicalTrials.gov number, NCT04427501.).
Copyright © 2020 Massachusetts Medical Society.
Figures

Comment in
-
Neutralizing Antibody LY-CoV555 for Outpatient Covid-19.N Engl J Med. 2021 Jan 14;384(2):189. doi: 10.1056/NEJMc2033787. Epub 2020 Dec 30. N Engl J Med. 2021. PMID: 33378610 No abstract available.
Similar articles
-
Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial.JAMA. 2021 Feb 16;325(7):632-644. doi: 10.1001/jama.2021.0202. JAMA. 2021. PMID: 33475701 Free PMC article. Clinical Trial.
-
REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19.N Engl J Med. 2021 Jan 21;384(3):238-251. doi: 10.1056/NEJMoa2035002. Epub 2020 Dec 17. N Engl J Med. 2021. PMID: 33332778 Free PMC article. Clinical Trial.
-
A Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19.N Engl J Med. 2021 Mar 11;384(10):905-914. doi: 10.1056/NEJMoa2033130. Epub 2020 Dec 22. N Engl J Med. 2021. PMID: 33356051 Free PMC article. Clinical Trial.
-
SARS-CoV-2-neutralising monoclonal antibodies to prevent COVID-19.Cochrane Database Syst Rev. 2022 Jun 17;6(6):CD014945. doi: 10.1002/14651858.CD014945.pub2. Cochrane Database Syst Rev. 2022. PMID: 35713300 Free PMC article. Review.
-
An overview of the preclinical discovery and development of bamlanivimab for the treatment of novel coronavirus infection (COVID-19): reasons for limited clinical use and lessons for the future.Expert Opin Drug Discov. 2021 Dec;16(12):1403-1414. doi: 10.1080/17460441.2021.1960819. Epub 2021 Jul 30. Expert Opin Drug Discov. 2021. PMID: 34304682 Free PMC article. Review.
Cited by
-
Disease Severity and Durability of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Response: A View Through the Lens of the Second Year of the Pandemic.Clin Infect Dis. 2021 Sep 15;73(6):e1345-e1347. doi: 10.1093/cid/ciab374. Clin Infect Dis. 2021. PMID: 33905478 Free PMC article. No abstract available.
-
Easing COVID-19 lockdown measures while protecting the older restricts the deaths to the level of the full lockdown.Sci Rep. 2021 Mar 12;11(1):5839. doi: 10.1038/s41598-021-82932-8. Sci Rep. 2021. PMID: 33712637 Free PMC article.
-
Electronic Health Record-based COVID-19 Interprofessional Case Collaboration.West J Emerg Med. 2022 Sep 14;23(5):789-791. doi: 10.5811/westjem.22.2.53886. West J Emerg Med. 2022. PMID: 36205668 Free PMC article. No abstract available.
-
Safety of mRNA-COVID-19 Vaccines in Patients With Thoracic Cancers.Clin Lung Cancer. 2023 Jan;24(1):e19-e26. doi: 10.1016/j.cllc.2022.10.004. Epub 2022 Oct 21. Clin Lung Cancer. 2023. PMID: 36372676 Free PMC article.
-
The time to offer treatments for COVID-19.Expert Opin Investig Drugs. 2021 May;30(5):505-518. doi: 10.1080/13543784.2021.1901883. Epub 2021 Apr 23. Expert Opin Investig Drugs. 2021. PMID: 33721548 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
