The combined usage of Matrine and Osthole inhibited endoplasmic reticulum apoptosis induced by PCV2
- PMID: 33046006
- PMCID: PMC7549248
- DOI: 10.1186/s12866-020-01986-2
The combined usage of Matrine and Osthole inhibited endoplasmic reticulum apoptosis induced by PCV2
Abstract
Background: Porcine circovirus type 2 (PCV2) is an important and common DNA virus that infect pig and can cause immunosuppression and induce apoptosis in the infected cells. To escape the host immune system, PCV2 constantly builds up complex mechanisms or mutates genes, and that is why it is difficult to eradicate complex PCV2 infection by relying on vaccines and single compound. At present, there is few literature reports on the effective prevention and treatment of PCV2 infection by a combination of two or more compounds. Previously, we have demonstrated the anti-PCV2 effect of Matrine in vitro, but its mechanism has not been further evaluated. Literatures have proven that Osthole has a variety of pharmacological activities, and we tested the ability of Osthole to inhibit PCV2 replication in cell culture. Therefore, this study explored the synergistic antiviral effect of Matrine combined with Osthole and their synergistic anti-apoptotic mechanism.
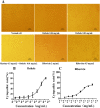
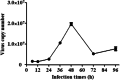
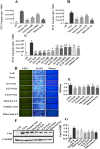
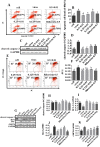
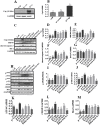
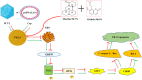
Results: Osthole alone had an anti-PCV2 effect, and then its synergistic anti-PCV2 effect of Osthole and Matrine was better than that of Matrine or Osthole alone as demonstrated by qRT-PCR, IFA and Western blotting results. The anti-apoptotic mechanism of these two compounds by inducing the PERK pathway by PCV2 was elucidated through Annexin V-FITC/PI, JC-1 and Western blotting. Matrine and Osthole combination could inhibit the expression of Cap in Cap-transfected PK-15 cells, thus inhibiting Cap-induced PERK apoptosis. Ribavirin was used as a positive control.
Conclusions: The combination of Osthole and Matrine had the synergistic effect of anti-PCV2 infection by directly inhibiting the expression of PCV2 Cap protein. The combination of these two compounds also inhibited PERK apoptosis induced by PCV2 Cap protein, possibly by regulating the level of GRP78. The results formed a base for further studies on the mechanism of anti-PCV2 in vivo using Matrine and Osthole combination and developing new anti-PCV2 compounds with Cap and GRP78 as therapeutic targets.
Keywords: Apoptosis; Cap; GRP78; Matrine; Osthole; PCV2.
Conflict of interest statement
All authors declared no competing conflict of interest.
Figures

Similar articles
-
Matrine combined with Osthole inhibited the PERK apoptosis of splenic lymphocytes in PCV2-infected mice model.BMC Vet Res. 2023 Jan 30;19(1):26. doi: 10.1186/s12917-023-03581-9. BMC Vet Res. 2023. PMID: 36717886 Free PMC article.
-
Porcine circovirus type 2 capsid protein induces unfolded protein response with subsequent activation of apoptosis.J Zhejiang Univ Sci B. 2017 Apr.;18(4):316-323. doi: 10.1631/jzus.B1600208. J Zhejiang Univ Sci B. 2017. PMID: 28378569 Free PMC article.
-
Matrine displayed antiviral activity in porcine alveolar macrophages co-infected by porcine reproductive and respiratory syndrome virus and porcine circovirus type 2.Sci Rep. 2016 Apr 15;6:24401. doi: 10.1038/srep24401. Sci Rep. 2016. PMID: 27080155 Free PMC article.
-
The Role of the PERK/eIF2α/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress.Curr Mol Med. 2016;16(6):533-44. doi: 10.2174/1566524016666160523143937. Curr Mol Med. 2016. PMID: 27211800 Free PMC article. Review.
-
Anti-tumor activities of matrine and oxymatrine: literature review.Tumour Biol. 2014 Jun;35(6):5111-9. doi: 10.1007/s13277-014-1680-z. Epub 2014 Feb 14. Tumour Biol. 2014. PMID: 24526416 Review.
Cited by
-
Matrine combined with Osthole inhibited the PERK apoptosis of splenic lymphocytes in PCV2-infected mice model.BMC Vet Res. 2023 Jan 30;19(1):26. doi: 10.1186/s12917-023-03581-9. BMC Vet Res. 2023. PMID: 36717886 Free PMC article.
-
Deoxynivalenol damages the intestinal barrier and biota of the broiler chickens.BMC Vet Res. 2022 Aug 15;18(1):311. doi: 10.1186/s12917-022-03392-4. BMC Vet Res. 2022. PMID: 35965338 Free PMC article.
-
The Antiviral Potential of Perilla frutescens: Advances and Perspectives.Molecules. 2024 Jul 15;29(14):3328. doi: 10.3390/molecules29143328. Molecules. 2024. PMID: 39064906 Free PMC article. Review.
-
A novel strategy for optimal component formula of anti-PRRSV from natural compounds using tandem mass tag labeled proteomic analyses.BMC Vet Res. 2022 May 14;18(1):179. doi: 10.1186/s12917-022-03184-w. BMC Vet Res. 2022. PMID: 35568854 Free PMC article.
References
-
- Tischer I, Gelderblom H, Vettermann W, Koch MA. A very small porcine virus with circular single-stranded DNA. Nature. 1982;295(5844):64–66. - PubMed
-
- Nawagitgul P, Morozov I, Bolin SR, Harms FA, Sorden SD, Paul PS. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J GenVirol. 2000;81(9):2281–2287. - PubMed
-
- Sinha A, Schalk S, Lager KM, Wang C, Opriessnig T. Singular PCV2a or PCV2b infection results in apoptosis of hepatocytes in clinically affected gnotobiotic pigs. Res Vet Sci. 2012;92(1):151–156. - PubMed
-
- Wei L, Zhu Z, Wang J, Zhang C, Quan R, Yan X. Regulatory role of ASK1 in porcine circovirus type 2-induced apoptosis. Virology. 2013;447(1–2):285–291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

