Cerebrovascular Senescence Is Associated With Tau Pathology in Alzheimer's Disease
- PMID: 33041998
- PMCID: PMC7525127
- DOI: 10.3389/fneur.2020.575953
Cerebrovascular Senescence Is Associated With Tau Pathology in Alzheimer's Disease
Abstract
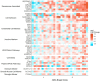
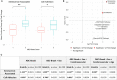
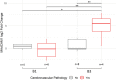
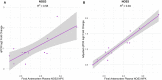
Alzheimer's Disease (AD) is associated with neuropathological changes, including aggregation of tau neurofibrillary tangles (NFTs) and amyloid-beta plaques. Mounting evidence indicates that vascular dysfunction also plays a key role in the pathogenesis and progression of AD, in part through endothelial dysfunction. Based on findings in animal models that tau pathology induces vascular abnormalities and cellular senescence, we hypothesized that tau pathology in the human AD brain leads to vascular senescence. To explore this hypothesis, we isolated intact microvessels from the dorsolateral prefrontal cortex (PFC, BA9) from 16 subjects with advanced Braak stages (Braak V/VI, B3) and 12 control subjects (Braak 0/I/II, B1), and quantified expression of 42 genes associated with senescence, cell adhesion, and various endothelial cell functions. Genes associated with endothelial senescence and leukocyte adhesion, including SERPINE1 (PAI-1), CXCL8 (IL8), CXCL1, CXCL2, ICAM-2, and TIE1, were significantly upregulated in B3 microvessels after adjusting for sex and cerebrovascular pathology. In particular, the senescence-associated secretory phenotype genes SERPINE1 and CXCL8 were upregulated by more than 2-fold in B3 microvessels after adjusting for sex, cerebrovascular pathology, and age at death. Protein quantification data from longitudinal plasma samples for a subset of 13 (n = 9 B3, n = 4 B1) subjects showed no significant differences in plasma senescence or adhesion-associated protein levels, suggesting that these changes were not associated with systemic vascular alterations. Future investigations of senescence biomarkers in both the peripheral and cortical vasculature could further elucidate links between tau pathology and vascular changes in human AD.
Keywords: Alzheimer's disease; endothelial senescence; gene expression; neurofibrillary tangles; plasma biomarkers; tau pathology; vascular dysfunction.
Copyright © 2020 Bryant, Hu, Carlyle, Arnold, Frosch, Das, Hyman and Bennett.
Figures


Similar articles
-
Longitudinal 18F-MK-6240 tau tangles accumulation follows Braak stages.Brain. 2021 Dec 16;144(11):3517-3528. doi: 10.1093/brain/awab248. Brain. 2021. PMID: 34515754 Free PMC article.
-
Tau pathology-dependent remodelling of cerebral arteries precedes Alzheimer's disease-related microvascular cerebral amyloid angiopathy.Acta Neuropathol. 2016 May;131(5):737-52. doi: 10.1007/s00401-016-1560-2. Epub 2016 Mar 17. Acta Neuropathol. 2016. PMID: 26988843 Free PMC article.
-
Transcriptomics and mechanistic elucidation of Alzheimer's disease risk genes in the brain and in vitro models.Neurobiol Aging. 2015 Feb;36(2):1221.e15-28. doi: 10.1016/j.neurobiolaging.2014.09.003. Epub 2014 Sep 6. Neurobiol Aging. 2015. PMID: 25281018
-
Spreading of amyloid, tau, and microvascular pathology in Alzheimer's disease: findings from neuropathological and neuroimaging studies.J Alzheimers Dis. 2014;42 Suppl 4:S421-9. doi: 10.3233/JAD-141461. J Alzheimers Dis. 2014. PMID: 25227313 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Meta-analyses of epigenetic age acceleration and GrimAge components of schizophrenia or first-episode psychosis.Schizophrenia (Heidelb). 2024 Nov 15;10(1):108. doi: 10.1038/s41537-024-00531-8. Schizophrenia (Heidelb). 2024. PMID: 39548083 Free PMC article.
-
Vascular contributions to cognitive impairment/dementia in diabetes: role of endothelial cells and pericytes.Am J Physiol Cell Physiol. 2022 Oct 1;323(4):C1177-C1189. doi: 10.1152/ajpcell.00072.2022. Epub 2022 Aug 29. Am J Physiol Cell Physiol. 2022. PMID: 36036445 Free PMC article. Review.
-
Biological aging processes underlying cognitive decline and neurodegenerative disease.J Clin Invest. 2022 May 16;132(10):e158453. doi: 10.1172/JCI158453. J Clin Invest. 2022. PMID: 35575089 Free PMC article. Review.
-
The landscape of human tissue and cell type specific expression and co-regulation of senescence genes.Mol Neurodegener. 2022 Jan 9;17(1):5. doi: 10.1186/s13024-021-00507-7. Mol Neurodegener. 2022. PMID: 35000600 Free PMC article.
-
Endothelin A receptors contribute to senescence of brain microvascular endothelial cells.Can J Physiol Pharmacol. 2022 Dec 1;100(12):1087-1096. doi: 10.1139/cjpp-2022-0071. Epub 2022 Nov 17. Can J Physiol Pharmacol. 2022. PMID: 36384316 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

