Activation of GABAB receptors results in excitatory modulation of calyx terminals in rat semicircular canal cristae
- PMID: 32816581
- PMCID: PMC7509296
- DOI: 10.1152/jn.00243.2020
Activation of GABAB receptors results in excitatory modulation of calyx terminals in rat semicircular canal cristae
Abstract
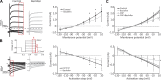
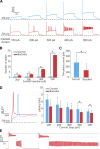
Previous studies have found GABA in vestibular end organs. However, existence of GABA receptors or possible GABAergic effects on vestibular nerve afferents has not been investigated. The current study was conducted to determine whether activation of GABAB receptors affects calyx afferent terminals in the central region of the cristae of semicircular canals. We used patch-clamp recording in postnatal day 13-18 (P13-P18) Sprague-Dawley rats of either sex. Application of GABAB receptor agonist baclofen inhibited voltage-sensitive potassium currents. This effect was blocked by selective GABAB receptor antagonist CGP 35348. Application of antagonists of small (SK)- and large-conductance potassium (BK) channels almost completely blocked the effects of baclofen. The remaining baclofen effect was blocked by cadmium chloride, suggesting that it could be due to inhibition of voltage-gated calcium channels. Furthermore, baclofen had no effect in the absence of calcium in the extracellular fluid. Inhibition of potassium currents by GABAB activation resulted in an excitatory effect on calyx terminal action potential firing. While in the control condition calyces could only fire a single action potential during step depolarizations, in the presence of baclofen they fired continuously during steps and a few even showed repetitive discharge. We also found a decrease in threshold for action potential generation and a decrease in first-spike latency during step depolarization. These results provide the first evidence for the presence of GABAB receptors on calyx terminals, showing that their activation results in an excitatory effect and that GABA inputs could be used to modulate calyx response properties.NEW & NOTEWORTHY Using in vitro whole cell patch-clamp recordings from calyx terminals in the vestibular end organs, we show that activation of GABAB receptors result in an excitatory effect, with decreased spike-frequency adaptation and shortened first-spike latencies. Our results suggest that these effects are mediated through inhibition of calcium-sensitive potassium channels.
Keywords: GABAB; calcium-sensitive potassium channel; efferent; supporting cell.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
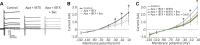

Similar articles
-
Persistent and resurgent Na+ currents in vestibular calyx afferents.J Neurophysiol. 2020 Aug 1;124(2):510-524. doi: 10.1152/jn.00124.2020. Epub 2020 Jul 15. J Neurophysiol. 2020. PMID: 32667253 Free PMC article.
-
GABAB receptor-mediated tonic inhibition of noradrenergic A7 neurons in the rat.J Neurophysiol. 2011 Jun;105(6):2715-28. doi: 10.1152/jn.00459.2010. Epub 2011 Mar 23. J Neurophysiol. 2011. PMID: 21430282
-
GABAB receptor-mediated responses in GABAergic projection neurones of rat nucleus reticularis thalami in vitro.J Physiol. 1996 Jun 15;493 ( Pt 3)(Pt 3):845-54. doi: 10.1113/jphysiol.1996.sp021427. J Physiol. 1996. PMID: 8799904 Free PMC article.
-
Activation of presynaptic GABAB receptors modulates GABAergic and glutamatergic inputs to the medial geniculate body.Hear Res. 2011 Oct;280(1-2):157-65. doi: 10.1016/j.heares.2011.05.018. Epub 2011 May 31. Hear Res. 2011. PMID: 21664264
-
Positive allosteric modulators of the GABAB receptor: a new class of ligands with therapeutic potential for alcohol use disorder.Alcohol Alcohol. 2024 Mar 16;59(3):agae018. doi: 10.1093/alcalc/agae018. Alcohol Alcohol. 2024. PMID: 38566580 Review.
Cited by
-
Measuring anxiety disorder in bipolar disorder using EVestG: broad impact of medication groups.Front Neurol. 2024 Jan 16;14:1303287. doi: 10.3389/fneur.2023.1303287. eCollection 2023. Front Neurol. 2024. PMID: 38292032 Free PMC article.
-
Editorial: Commonalities and Differences in Vestibular and Auditory Pathways.Front Neurosci. 2022 Mar 23;16:876798. doi: 10.3389/fnins.2022.876798. eCollection 2022. Front Neurosci. 2022. PMID: 35401079 Free PMC article. No abstract available.
-
Cell-Type Specific Inhibition Controls the High-Frequency Oscillations in the Medial Entorhinal Cortex.Int J Mol Sci. 2022 Nov 15;23(22):14087. doi: 10.3390/ijms232214087. Int J Mol Sci. 2022. PMID: 36430563 Free PMC article.
-
Dopaminergic Inhibition of Na+ Currents in Vestibular Inner Ear Afferents.Front Neurosci. 2021 Sep 9;15:710321. doi: 10.3389/fnins.2021.710321. eCollection 2021. Front Neurosci. 2021. PMID: 34580582 Free PMC article.
-
The mammalian efferent vestibular system utilizes cholinergic mechanisms to excite primary vestibular afferents.Sci Rep. 2021 Jan 13;11(1):1231. doi: 10.1038/s41598-020-80367-1. Sci Rep. 2021. PMID: 33441862 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

