Melatonin suppresses chronic restraint stress-mediated metastasis of epithelial ovarian cancer via NE/AKT/β-catenin/SLUG axis
- PMID: 32811805
- PMCID: PMC7435194
- DOI: 10.1038/s41419-020-02906-y
Melatonin suppresses chronic restraint stress-mediated metastasis of epithelial ovarian cancer via NE/AKT/β-catenin/SLUG axis
Erratum in
-
Correction: Melatonin suppresses chronic restraint stress-mediated metastasis of epithelial ovarian cancer via NE/AKT/β-catenin/SLUG axis.Cell Death Dis. 2020 Sep 8;11(9):726. doi: 10.1038/s41419-020-02958-0. Cell Death Dis. 2020. PMID: 32901009 Free PMC article.
Abstract
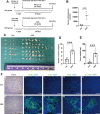
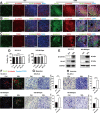
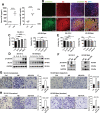
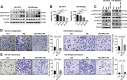
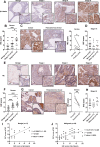
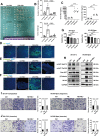
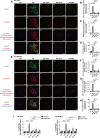
Chronic stress has been shown to facilitate progression of epithelial ovarian cancer (EOC), however, the neuro-endocranial mechanism participating in this process still remains unclear. Here, we reported that chronic restraint stress (CRS) promoted the abdominal implantation metastasis of EOC cells and the expression of epithelial-mesenchymal transition-related markers in tumor-bearing mouse model, including TWIST, SLUG, SNAIL, and β-catenin. We observed that β-catenin co-expressed with SLUG and norepinephrine (NE) in tumor tissues obtained from nude mice. Further ex vivo experiments revealed that NE promoted migration and invasion of ovarian cancer cells and SLUG expression through upregulating expression and improving transcriptional function of β-catenin in vitro. A human phosphor-kinase array suggested that NE activated various kinases in ovarian cancer cells, and we further confirmed that AKT inhibitor reduced NE-mediated pro-metastatic impacts and activation of the β-catenin/SLUG axis. Furthermore, the expression levels of NE and β-catenin were examined in ovarian tumor tissues by using tumor tissue arrays. Results showed that the expression levels of both NE and β-catenin were associated with poor clinical stage of serous EOC. Moreover, we found that melatonin (MLT) effectively reduced the abdominal tumor burden of ovarian cancer induced by CRS, which was partially related to the inhibition of the NE/AKT/β-catenin/SLUG axis. Collectively, these findings suggest a novel mechanism for CRS-mediated ovarian cancer metastasis and MLT has a potential therapeutic efficacy against ovarian cancer.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Nobiletin inhibits invasion via inhibiting AKT/GSK3β/β-catenin signaling pathway in Slug-expressing glioma cells.Oncol Rep. 2017 May;37(5):2847-2856. doi: 10.3892/or.2017.5522. Epub 2017 Mar 23. Oncol Rep. 2017. PMID: 28339056
-
Emodin Inhibits the Epithelial to Mesenchymal Transition of Epithelial Ovarian Cancer Cells via ILK/GSK-3β/Slug Signaling Pathway.Biomed Res Int. 2016;2016:6253280. doi: 10.1155/2016/6253280. Epub 2016 Dec 20. Biomed Res Int. 2016. PMID: 28097141 Free PMC article.
-
BTB and CNC homology 1 (Bach1) promotes human ovarian cancer cell metastasis by HMGA2-mediated epithelial-mesenchymal transition.Cancer Lett. 2019 Mar 31;445:45-56. doi: 10.1016/j.canlet.2019.01.003. Epub 2019 Jan 14. Cancer Lett. 2019. PMID: 30654010
-
Snail transcription factors - Characteristics, regulation and molecular targets relevant in vital cellular activities of ovarian cancer cells.Biochim Biophys Acta Mol Cell Res. 2024 Jun;1871(5):119705. doi: 10.1016/j.bbamcr.2024.119705. Epub 2024 Mar 19. Biochim Biophys Acta Mol Cell Res. 2024. PMID: 38513918 Review.
-
Adipocytes: active facilitators in epithelial ovarian cancer progression?J Ovarian Res. 2020 Sep 23;13(1):115. doi: 10.1186/s13048-020-00718-4. J Ovarian Res. 2020. PMID: 32967712 Free PMC article. Review.
Cited by
-
An updated review of mechanistic potentials of melatonin against cancer: pivotal roles in angiogenesis, apoptosis, autophagy, endoplasmic reticulum stress and oxidative stress.Cancer Cell Int. 2021 Mar 31;21(1):188. doi: 10.1186/s12935-021-01892-1. Cancer Cell Int. 2021. PMID: 33789681 Free PMC article. Review.
-
Investigating the crosstalk between chronic stress and immune cells: implications for enhanced cancer therapy.Front Neurosci. 2023 Nov 28;17:1321176. doi: 10.3389/fnins.2023.1321176. eCollection 2023. Front Neurosci. 2023. PMID: 38089966 Free PMC article. Review.
-
Mechanisms of Melatonin in Obesity: A Review.Int J Mol Sci. 2021 Dec 25;23(1):218. doi: 10.3390/ijms23010218. Int J Mol Sci. 2021. PMID: 35008644 Free PMC article. Review.
-
An Expanded Narrative Review of Neurotransmitters on Alzheimer's Disease: The Role of Therapeutic Interventions on Neurotransmission.Mol Neurobiol. 2024 Jul 16. doi: 10.1007/s12035-024-04333-y. Online ahead of print. Mol Neurobiol. 2024. PMID: 39012443 Review.
-
Inhibition of FSTL3 abates the proliferation and metastasis of renal cell carcinoma via the GSK-3β/β-catenin signaling pathway.Aging (Albany NY). 2021 Sep 23;13(18):22528-22543. doi: 10.18632/aging.203564. Epub 2021 Sep 23. Aging (Albany NY). 2021. PMID: 34555811 Free PMC article.
References
-
- Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–1388. - PubMed
-
- Krizanova O, Babula P, Pacak K. Stress, catecholaminergic system and cancer. Stress. 2016;19:419–428. - PubMed
-
- Lutgendorf SK, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin. Cancer Res. 2003;9:4514–4521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

