Bloodstream infections in critically ill patients with COVID-19
- PMID: 32535894
- PMCID: PMC7323143
- DOI: 10.1111/eci.13319
Bloodstream infections in critically ill patients with COVID-19
Abstract
Background: Little is known about the incidence and risk of intensive care unit (ICU)-acquired bloodstream infections (BSI) in critically ill patients with coronavirus disease 2019 (COVID-19).
Materials and methods: This retrospective, single-centre study was conducted in Northern Italy. The primary study objectives were as follows: (a) to assess the incidence rate of ICU-acquired BSI and (b) to assess the cumulative risk of developing ICU-acquired BSI.
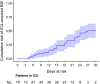
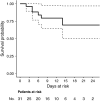
Results: Overall, 78 critically ill patients with COVID-19 were included in the study. Forty-five episodes of ICU-acquired BSI were registered in 31 patients, with an incidence rate of 47 episodes (95% confidence interval [CI] 35-63) per 1000 patient-days at risk. The estimated cumulative risk of developing at least one BSI episode was of almost 25% after 15 days at risk and possibly surpassing 50% after 30 days at risk. In multivariable analysis, anti-inflammatory treatment was independently associated with the development of BSI (cause-specific hazard ratio [csHR] 1.07 with 95% CI 0.38-3.04 for tocilizumab, csHR 3.95 with 95% CI 1.20-13.03 for methylprednisolone and csHR 10.69 with 95% CI 2.71-42.17 for methylprednisolone plus tocilizumab, with no anti-inflammatory treatment as the reference group; overall P for the dummy variable = 0.003).
Conclusions: The incidence rate of BSI was high, and the cumulative risk of developing BSI increased with ICU stay. Further study will clarify if the increased risk of BSI we detected in COVID-19 patients treated with anti-inflammatory drugs is outweighed by the benefits of reducing any possible pro-inflammatory dysregulation induced by SARS-CoV-2.
Keywords: BSI; COVID-19; SARS-CoV-2; coronavirus; steroid; tocilizumab.
© 2020 Stichting European Society for Clinical Investigation Journal Foundation.
Conflict of interest statement
Outside the submitted work, DR Giacobbe reports honoraria from Stepstone Pharma GmbH and unconditional grants from MSD Italia and Correvio Italia. Outside the submitted work, M. Bassetti has received funding for scientific advisory boards, travel and speaker honoraria from Angelini, Astellas, AstraZeneca, Basilea, Bayer, BioMèrieux, Cidara, Correvio, Cubist, Menarini, Molteni, MSD, Nabriva, Paratek, Pfizer, Roche, Shionogi, Tetraphase, Thermo Fisher and The Medicine Company.
Figures
Similar articles
-
Enterococcal bloodstream infections in critically ill patients with COVID-19: a case series.Ann Med. 2021 Dec;53(1):1779-1786. doi: 10.1080/07853890.2021.1988695. Ann Med. 2021. PMID: 34637370 Free PMC article.
-
Protective effect of SARS-CoV-2 preventive measures against ESKAPE and Escherichia coli infections.Eur J Clin Invest. 2021 Dec;51(12):e13687. doi: 10.1111/eci.13687. Epub 2021 Oct 11. Eur J Clin Invest. 2021. PMID: 34599600 Free PMC article.
-
Tocilizumab and steroid treatment in patients with COVID-19 pneumonia.PLoS One. 2020 Aug 20;15(8):e0237831. doi: 10.1371/journal.pone.0237831. eCollection 2020. PLoS One. 2020. PMID: 32817707 Free PMC article.
-
Application of Chinese Medicine in the Management of Critical Conditions: A Review on Sepsis.Am J Chin Med. 2020;48(6):1315-1330. doi: 10.1142/S0192415X20500640. Epub 2020 Sep 9. Am J Chin Med. 2020. PMID: 32907362 Review.
-
Balancing evidence and frontline experience in the early phases of the COVID-19 pandemic: current position of the Italian Society of Anti-infective Therapy (SITA) and the Italian Society of Pulmonology (SIP).Clin Microbiol Infect. 2020 Jul;26(7):880-894. doi: 10.1016/j.cmi.2020.04.031. Epub 2020 Apr 29. Clin Microbiol Infect. 2020. PMID: 32360444 Free PMC article. Review.
Cited by
-
Acute Gastrointestinal Injury and Feeding Intolerance as Prognostic Factors in Critically Ill COVID-19 Patients.J Gastrointest Surg. 2022 Jan;26(1):181-190. doi: 10.1007/s11605-021-05015-z. Epub 2021 Apr 27. J Gastrointest Surg. 2022. PMID: 33905039 Free PMC article.
-
Bacterial and fungal superinfections in critically ill patients with COVID-19.Intensive Care Med. 2020 Nov;46(11):2071-2074. doi: 10.1007/s00134-020-06219-8. Epub 2020 Sep 9. Intensive Care Med. 2020. PMID: 32902729 Free PMC article. No abstract available.
-
Therapeutic Efficacy and Outcomes of Remdesivir versus Remdesivir with Tocilizumab in Severe SARS-CoV-2 Infection.Int J Mol Sci. 2022 Nov 21;23(22):14462. doi: 10.3390/ijms232214462. Int J Mol Sci. 2022. PMID: 36430945 Free PMC article.
-
Hospital-acquired bloodstream infections in patients deceased with COVID-19 in Italy (2020-2021).Front Med (Lausanne). 2022 Nov 17;9:1041668. doi: 10.3389/fmed.2022.1041668. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36465906 Free PMC article.
-
Vascular occlusion by neutrophil extracellular traps in COVID-19.EBioMedicine. 2020 Aug;58:102925. doi: 10.1016/j.ebiom.2020.102925. Epub 2020 Jul 31. EBioMedicine. 2020. PMID: 32745993 Free PMC article.
References
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

