miR-26a regulates extracellular vesicle secretion from prostate cancer cells via targeting SHC4, PFDN4, and CHORDC1
- PMID: 32494663
- PMCID: PMC7190312
- DOI: 10.1126/sciadv.aay3051
miR-26a regulates extracellular vesicle secretion from prostate cancer cells via targeting SHC4, PFDN4, and CHORDC1
Abstract
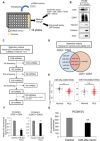
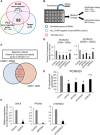
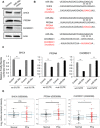
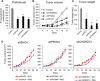
Extracellular vesicles (EVs) are involved in intercellular communication during cancer progression; thus, elucidating the mechanism of EV secretion in cancer cells will contribute to the development of an EV-targeted cancer treatment. However, the biogenesis of EVs in cancer cells is not fully understood. MicroRNAs (miRNAs) regulate a variety of biological phenomena; thus, miRNAs could regulate EV secretion. Here, we performed high-throughput miRNA-based screening to identify the regulators of EV secretion using an ExoScreen assay. By using this method, we identified miR-26a involved in EV secretion from prostate cancer (PCa) cells. In addition, we found that SHC4, PFDN4, and CHORDC1 genes regulate EV secretion in PCa cells. Furthermore, the progression of the PCa cells suppressing these genes was inhibited in an in vivo study. Together, our findings suggest that miR-26a regulates EV secretion via targeting SHC4, PFDN4, and CHORDC1 in PCa cells, resulting in the suppression of PCa progression.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures
Similar articles
-
The miR-1908/SRM regulatory axis contributes to extracellular vesicle secretion in prostate cancer.Cancer Sci. 2020 Sep;111(9):3258-3267. doi: 10.1111/cas.14535. Epub 2020 Jul 18. Cancer Sci. 2020. PMID: 32558033 Free PMC article.
-
Exosomal miRNAs from Prostate Cancer Impair Osteoblast Function in Mice.Int J Mol Sci. 2022 Jan 24;23(3):1285. doi: 10.3390/ijms23031285. Int J Mol Sci. 2022. PMID: 35163219 Free PMC article.
-
Impact of Extracellular Vesicle Isolation Methods on Downstream Mirna Analysis in Semen: A Comparative Study.Int J Mol Sci. 2020 Aug 19;21(17):5949. doi: 10.3390/ijms21175949. Int J Mol Sci. 2020. PMID: 32824915 Free PMC article.
-
Communication of prostate cancer cells with bone cells via extracellular vesicle RNA; a potential mechanism of metastasis.Oncogene. 2019 Mar;38(10):1751-1763. doi: 10.1038/s41388-018-0540-5. Epub 2018 Oct 23. Oncogene. 2019. PMID: 30353168 Free PMC article. Review.
-
Application of extracellular vesicles in the diagnosis and treatment of prostate cancer: Implications for clinical practice.Crit Rev Oncol Hematol. 2021 Nov;167:103495. doi: 10.1016/j.critrevonc.2021.103495. Epub 2021 Oct 13. Crit Rev Oncol Hematol. 2021. PMID: 34655743 Review.
Cited by
-
The miR-1908/SRM regulatory axis contributes to extracellular vesicle secretion in prostate cancer.Cancer Sci. 2020 Sep;111(9):3258-3267. doi: 10.1111/cas.14535. Epub 2020 Jul 18. Cancer Sci. 2020. PMID: 32558033 Free PMC article.
-
SHC4 promotes tumor proliferation and metastasis by activating STAT3 signaling in hepatocellular carcinoma.Cancer Cell Int. 2022 Jan 15;22(1):24. doi: 10.1186/s12935-022-02446-9. Cancer Cell Int. 2022. PMID: 35033067 Free PMC article.
-
miR-26a is a Key Therapeutic Target with Enormous Potential in the Diagnosis and Prognosis of Human Disease.Curr Med Chem. 2024;31(18):2550-2570. doi: 10.2174/0109298673271808231116075056. Curr Med Chem. 2024. PMID: 38204224 Review.
-
miR-15a targets the HSP90 co-chaperone Morgana in chronic myeloid leukemia.Sci Rep. 2024 Jul 2;14(1):15089. doi: 10.1038/s41598-024-65404-7. Sci Rep. 2024. PMID: 38956394 Free PMC article.
-
Exosomal miRNAs-a diagnostic biomarker acting as a guiding light in the diagnosis of prostate cancer.Funct Integr Genomics. 2022 Dec 27;23(1):23. doi: 10.1007/s10142-022-00951-8. Funct Integr Genomics. 2022. PMID: 36574059 Review.
References
-
- Denzer K., Kleijmeer M. J., Heijnen H. F., Stoorvogel W., Geuze H. J., Exosome: From internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 113, 3365–3374 (2000). - PubMed
-
- Fauré J., Lachenal G., Court M., Hirrlinger J., Chatellard-Causse C., Blot B., Grange J., Schoehn G., Goldberg Y., Boyer V., Kirchhoff F., Raposo G., Garin J., Sadoul R., Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 31, 642–648 (2006). - PubMed
-
- Hosseini-Beheshti E., Choi W., Weiswald L.-B., Kharmate G., Ghaffari M., Roshan-Moniri M., Hassona M. D., Chan L., Chin M. Y., Tai I. T., Rennie P. S., Fazli L., Tomlinson Guns E. S., Exosomes confer pro-survival signals to alter the phenotype of prostate cells in their surrounding environment. Oncotarget 7, 14639–14658 (2016). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

