Donor monocyte-derived macrophages promote human acute graft-versus-host disease
- PMID: 32453711
- PMCID: PMC7456218
- DOI: 10.1172/JCI133909
Donor monocyte-derived macrophages promote human acute graft-versus-host disease
Abstract
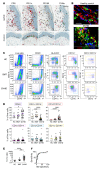
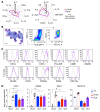
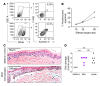
Myelopoiesis is invariably present and contributes to pathology in animal models of graft-versus-host disease (GVHD). In humans, a rich inflammatory infiltrate bearing macrophage markers has also been described in histological studies. In order to determine the origin, functional properties, and role in pathogenesis of these cells, we isolated single-cell suspensions from acute cutaneous GVHD and subjected them to genotype, transcriptome, and in vitro functional analysis. A donor-derived population of CD11c+CD14+ cells was the dominant population of all leukocytes in GVHD. Surface phenotype and NanoString gene expression profiling indicated the closest steady-state counterpart of these cells to be monocyte-derived macrophages. In GVHD, however, there was upregulation of monocyte antigens SIRPα and S100A8/9 transcripts associated with leukocyte trafficking, pattern recognition, antigen presentation, and costimulation. Isolated GVHD macrophages stimulated greater proliferation and activation of allogeneic T cells and secreted higher levels of inflammatory cytokines than their steady-state counterparts. In HLA-matched mixed leukocyte reactions, we also observed differentiation of activated macrophages with a similar phenotype. These exhibited cytopathicity to a keratinocyte cell line and mediated pathological damage to skin explants independently of T cells. Together, these results define the origin, functional properties, and potential pathogenic roles of human GVHD macrophages.
Keywords: Bone marrow transplantation; Immunology; Macrophages; Stem cell transplantation; Transplantation.
Conflict of interest statement
Figures




Comment in
-
Alternative mechanisms that mediate graft-versus-host disease in allogeneic hematopoietic cell transplants.J Clin Invest. 2020 Sep 1;130(9):4532-4535. doi: 10.1172/JCI140064. J Clin Invest. 2020. PMID: 32716364 Free PMC article.
Similar articles
-
Tissue Damage Caused by Myeloablative, but Not Non-Myeloablative, Conditioning before Allogeneic Stem Cell Transplantation Results in Dermal Macrophage Recruitment without Active T-Cell Interaction.Front Immunol. 2018 Feb 27;9:331. doi: 10.3389/fimmu.2018.00331. eCollection 2018. Front Immunol. 2018. PMID: 29535719 Free PMC article. Clinical Trial.
-
Murine sclerodermatous graft-versus-host disease, a model for human scleroderma: cutaneous cytokines, chemokines, and immune cell activation.J Immunol. 2002 Mar 15;168(6):3088-98. doi: 10.4049/jimmunol.168.6.3088. J Immunol. 2002. PMID: 11884483
-
Peripheral host T cells survive hematopoietic stem cell transplantation and promote graft-versus-host disease.J Clin Invest. 2020 Sep 1;130(9):4624-4636. doi: 10.1172/JCI129965. J Clin Invest. 2020. PMID: 32516138 Free PMC article. Clinical Trial.
-
Acute graft-vs-host disease: pathobiology and management.Exp Hematol. 2001 Mar;29(3):259-77. doi: 10.1016/s0301-472x(00)00677-9. Exp Hematol. 2001. PMID: 11274753 Review.
-
Macrophage-mediated complications after stem cell transplantation.Pathol Int. 2019 Dec;69(12):679-687. doi: 10.1111/pin.12865. Epub 2019 Oct 16. Pathol Int. 2019. PMID: 31621148 Review.
Cited by
-
Monocytes as an early risk factor for acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation.Front Immunol. 2024 Sep 12;15:1433091. doi: 10.3389/fimmu.2024.1433091. eCollection 2024. Front Immunol. 2024. PMID: 39328417 Free PMC article.
-
Immunopathological mechanisms and clinical manifestations of ocular graft-versus-host disease following hematopoietic stem cell transplantation.Bone Marrow Transplant. 2024 Aug;59(8):1049-1056. doi: 10.1038/s41409-024-02321-3. Epub 2024 May 31. Bone Marrow Transplant. 2024. PMID: 38822141 Review.
-
A genome-wide association study on hematopoietic stem cell transplantation reveals novel genomic loci associated with transplant outcomes.Front Immunol. 2024 Feb 7;15:1280876. doi: 10.3389/fimmu.2024.1280876. eCollection 2024. Front Immunol. 2024. PMID: 38384455 Free PMC article.
-
ROCK1/2 signaling contributes to corticosteroid-refractory acute graft-versus-host disease.Nat Commun. 2024 Jan 10;15(1):446. doi: 10.1038/s41467-024-44703-7. Nat Commun. 2024. PMID: 38199985 Free PMC article.
-
Diverse macrophage populations contribute to distinct manifestations of human cutaneous graft-versus-host disease.Br J Dermatol. 2024 Feb 16;190(3):402-414. doi: 10.1093/bjd/ljad402. Br J Dermatol. 2024. PMID: 38010706 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

