Luteolin-7-O-Glucoside Inhibits Oral Cancer Cell Migration and Invasion by Regulating Matrix Metalloproteinase-2 Expression and Extracellular Signal-Regulated Kinase Pathway
- PMID: 32224968
- PMCID: PMC7226481
- DOI: 10.3390/biom10040502
Luteolin-7-O-Glucoside Inhibits Oral Cancer Cell Migration and Invasion by Regulating Matrix Metalloproteinase-2 Expression and Extracellular Signal-Regulated Kinase Pathway
Abstract
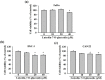
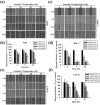
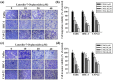
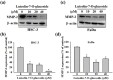
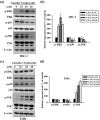
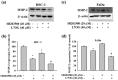
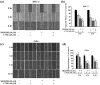
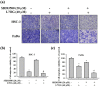
Oral squamous cell carcinoma is the sixth most common type of cancer globally, which is associated with high rates of cancer-related deaths. Metastasis to distant organs is the main reason behind worst prognostic outcome of oral cancer. In the present study, we aimed at evaluating the effects of a natural plant flavonoid, luteolin-7-O-glucoside, on oral cancer cell migration and invasion. The study findings showed that in addition to preventing cell proliferation, luteolin-7-O-glucoside caused a significant reduction in oral cancer cell migration and invasion. Mechanistically, luteolin-7-O-glucoside caused a reduction in cancer metastasis by reducing p38 phosphorylation and downregulating matrix metalloproteinase (MMP)-2 expression. Using a p38 inhibitor, SB203580, we proved that luteolin-7-O-glucoside exerts anti-migratory effects by suppressing p38-mediated increased expression of MMP-2. This is the first study to demonstrate the luteolin-7-O-glucoside inhibits cell migration and invasion by regulating MMP-2 expression and extracellular signal-regulated kinase pathway in human oral cancer cell. The study identifies luteolin-7-O-glucoside as a potential anti-cancer candidate that can be utilized clinically for improving oral cancer prognosis.
Keywords: Luteolin-7-O-glucoside; MMP-2; invasion; migration; oral cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Pinostilbene Hydrate Suppresses Human Oral Cancer Cell Metastasis by Downregulation of Matrix Metalloproteinase-2 Through the Mitogen-Activated Protein Kinase Signaling Pathway.Cell Physiol Biochem. 2018;50(3):911-923. doi: 10.1159/000494476. Epub 2018 Oct 24. Cell Physiol Biochem. 2018. PMID: 30355929
-
Naringin inhibits the invasion and migration of human glioblastoma cell via downregulation of MMP-2 and MMP-9 expression and inactivation of p38 signaling pathway.Tumour Biol. 2016 Mar;37(3):3831-9. doi: 10.1007/s13277-015-4230-4. Epub 2015 Oct 16. Tumour Biol. 2016. PMID: 26474590
-
Epigallocatechin gallate sensitizes CAL-27 human oral squamous cell carcinoma cells to the anti-metastatic effects of gefitinib (Iressa) via synergistic suppression of epidermal growth factor receptor and matrix metalloproteinase-2.Oncol Rep. 2012 Nov;28(5):1799-807. doi: 10.3892/or.2012.1991. Epub 2012 Aug 24. Oncol Rep. 2012. PMID: 22923287
-
Advancing oral health: Harnessing the potential of chitosan and polyphenols in innovative mouthwash formulation.Biomed Pharmacother. 2024 Jun;175:116654. doi: 10.1016/j.biopha.2024.116654. Epub 2024 Apr 30. Biomed Pharmacother. 2024. PMID: 38692066 Review.
-
Luteolin as a potential therapeutic candidate for lung cancer: Emerging preclinical evidence.Biomed Pharmacother. 2024 Jul;176:116909. doi: 10.1016/j.biopha.2024.116909. Epub 2024 Jun 8. Biomed Pharmacother. 2024. PMID: 38852513 Review.
Cited by
-
Advances in Small Molecular Agents against Oral Cancer.Molecules. 2024 Apr 3;29(7):1594. doi: 10.3390/molecules29071594. Molecules. 2024. PMID: 38611874 Free PMC article. Review.
-
Luteolin, a Potent Anticancer Compound: From Chemistry to Cellular Interactions and Synergetic Perspectives.Cancers (Basel). 2022 Oct 31;14(21):5373. doi: 10.3390/cancers14215373. Cancers (Basel). 2022. PMID: 36358791 Free PMC article. Review.
-
Metabolomics-Based Analyses of Dynamic Changes in Flavonoid Profiles in the Black Mulberry Winemaking Process.Foods. 2023 May 31;12(11):2221. doi: 10.3390/foods12112221. Foods. 2023. PMID: 37297465 Free PMC article.
-
Cynaroside Induces G1 Cell Cycle Arrest by Downregulating Cell Division Cycle 25A in Colorectal Cancer.Molecules. 2024 Mar 28;29(7):1508. doi: 10.3390/molecules29071508. Molecules. 2024. PMID: 38611789 Free PMC article.
-
Phytotherapeutics in Cancer: From Potential Drug Candidates to Clinical Translation.Curr Top Med Chem. 2024;24(12):1050-1074. doi: 10.2174/0115680266282518231231075311. Curr Top Med Chem. 2024. PMID: 38279745 Review.
References
-
- Smriti K., Ray M., Chatterjee T., Shenoy R.-P., Gadicherla S., Pentapati K.-C., Rustaqi N. Salivary MMP-9 as a Biomarker for the Diagnosis of Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2020;21:233–238. doi: 10.31557/APJCP.2020.21.1.233. - DOI - PMC - PubMed
-
- Su S.-C., Lin C.-W., Liu Y.-F., Fan W.-L., Chen M.-K., Yu C.-P., Yang W.-E., Su C.-W., Chuang C.-Y., Li W.-H., et al. Exome Sequencing of Oral Squamous Cell Carcinoma Reveals Molecular Subgroups and Novel Therapeutic Opportunities. Theranostics. 2017;7:1088–1099. doi: 10.7150/thno.18551. - DOI - PMC - PubMed
-
- Haigentz M., Jr., Hartl D.M., Silver C.E., Langendijk J.A., Strojan P., Paleri V., De Bree R., Machiels J.P., Hamoir M., Rinaldo A., et al. Distant metastases from head and neck squamous cell carcinoma. Part III. Treatment. Oral Oncol. 2012;48:787–793. doi: 10.1016/j.oraloncology.2012.03.019. - DOI - PubMed
-
- Takes R.P., Rinaldo A., Silver C.E., Haigentz M., Jr., Woolgar J.A., Triantafyllou A., Mondin V., Paccagnella D., De Bree R., Shaha A.R., et al. Distant metastases from head and neck squamous cell carcinoma. Part I. Basic aspects. Oral Oncol. 2012;48:775–779. doi: 10.1016/j.oraloncology.2012.03.013. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

