Memory T cells: strategies for optimizing tumor immunotherapy
- PMID: 32221812
- PMCID: PMC7381543
- DOI: 10.1007/s13238-020-00707-9
Memory T cells: strategies for optimizing tumor immunotherapy
Abstract
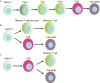
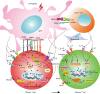
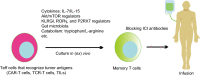
Several studies have demonstrated that memory T cells including stem cell memory (Tscm) T cells and central memory (Tcm) T cells show superior persistence and antitumor immunity compared with effector memory T (Tem) cells and effector T (Teff) cells. Furthermore, the Tcm/Teff ratio has been reported to be a predictive biomarker of immune responses against some tumors. Thus, a system-level understanding of the mechanisms underlying the differentiation of effector and memory T cells is of increasing importance for developing immunological strategies against various tumors. This review focuses on recent advances in efficacy against tumors, the origin, formation mechanisms of memory T cells, and the role of the gut microbiota in memory T cell formation. Furthermore, we summarize strategies to generate memory T cells in (ex) vivo that, might be applicable in clinical practice.
Keywords: gut microbiota; memory T cells; metabolism; tumor immunology.
Figures
Similar articles
-
T-memory cells against cancer: Remembering the enemy.Cell Immunol. 2019 Apr;338:27-31. doi: 10.1016/j.cellimm.2019.03.002. Epub 2019 Mar 16. Cell Immunol. 2019. PMID: 30928016 Review.
-
Memory T cell, exhaustion, and tumor immunity.Immunol Med. 2020 Mar;43(1):1-9. doi: 10.1080/25785826.2019.1698261. Epub 2019 Dec 10. Immunol Med. 2020. PMID: 31822213 Review.
-
Generation and application of human induced-stem cell memory T cells for adoptive immunotherapy.Cancer Sci. 2018 Jul;109(7):2130-2140. doi: 10.1111/cas.13648. Epub 2018 Jun 28. Cancer Sci. 2018. PMID: 29790621 Free PMC article.
-
Human effector T cells derived from central memory cells rather than CD8(+)T cells modified by tumor-specific TCR gene transfer possess superior traits for adoptive immunotherapy.Cancer Lett. 2013 Oct 10;339(2):195-207. doi: 10.1016/j.canlet.2013.06.009. Epub 2013 Jun 18. Cancer Lett. 2013. PMID: 23791878
-
Role of memory T cell subsets for adoptive immunotherapy.Semin Immunol. 2016 Feb;28(1):28-34. doi: 10.1016/j.smim.2016.02.001. Epub 2016 Mar 11. Semin Immunol. 2016. PMID: 26976826 Free PMC article. Review.
Cited by
-
Metabolic reprogramming involves in transition of activated/resting CD4+ memory T cells and prognosis of gastric cancer.Front Immunol. 2023 Nov 27;14:1275461. doi: 10.3389/fimmu.2023.1275461. eCollection 2023. Front Immunol. 2023. PMID: 38090588 Free PMC article.
-
ZYG11B participates in the modulation of colorectal cancer cell proliferation and immune infiltration and is a prognostic biomarker.BMC Cancer. 2024 Sep 30;24(1):1203. doi: 10.1186/s12885-024-12963-7. BMC Cancer. 2024. PMID: 39350118 Free PMC article.
-
Antitumor activity of T cells secreting αCD133-αCD3 bispecific T-cell engager against cholangiocarcinoma.PLoS One. 2022 Mar 21;17(3):e0265773. doi: 10.1371/journal.pone.0265773. eCollection 2022. PLoS One. 2022. PMID: 35312724 Free PMC article.
-
Enhancing T cell anti-tumor efficacy with a PD1-TIGIT chimeric immune-checkpoint switch receptor.Oncoimmunology. 2023 Oct 5;12(1):2265703. doi: 10.1080/2162402X.2023.2265703. eCollection 2023. Oncoimmunology. 2023. PMID: 37808405 Free PMC article.
-
The immune response behind peptide vaccination in diffuse midline glioma.Mol Oncol. 2024 Aug;18(8):1849-1852. doi: 10.1002/1878-0261.13686. Epub 2024 Jun 16. Mol Oncol. 2024. PMID: 38880657 Free PMC article.
References
-
- Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, Tamada K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol. 2018;36:346–351. - PubMed
-
- Ahlers JD, Belyakov IM, Terabe M, Koka R, Donaldson DD, Thomas EK, Berzofsky JA. A push-pull approach to maximize vaccine efficacy: abrogating suppression with an IL-13 inhibitor while augmenting help with granulocyte/macrophage colony-stimulating factor and CD40L. Proc Natl Acad Sci U S A. 2002;99:13020–13025. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

