No evidence for hypogammaglobulinemia in patients with paroxysmal nocturnal hemoglobinuria (PNH) chronically treated with ravulizumab
- PMID: 32218584
- PMCID: PMC7101163
- DOI: 10.1371/journal.pone.0230869
No evidence for hypogammaglobulinemia in patients with paroxysmal nocturnal hemoglobinuria (PNH) chronically treated with ravulizumab
Abstract
Introduction: Ravulizumab (ALXN1210) is a long-lasting recycling IgG monoclonal antibody with an increased affinity for the neonatal Fc receptor (FcRn). The FcRn is essential for regulating IgG homeostasis. Saturation of the FcRn pathway is seen under high IgG doses as they compete with endogenous IgG to bind the FcRn by their Fc regions, resulting in enhanced IgG clearance.
Patients/methods: Between Jan 2016 and Jun 2019 (median observation time 21.6 months (6-37.7 months)) serum IgG concentrations and IgG1-4 subclasses were evaluated over a longitudinal course (post-hoc analysis) in 12 ravulizumab-treated adult patients with paroxysmal nocturnal hemoglobinuria (PNH) (58% (7/12) males, median age 50 years (yrs) (18-70 yrs)). All patients were enrolled in one of the three ravulizumab-PNH-related trials (201-, 301-, or 302-study) at the University Hospital Essen.
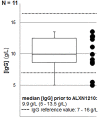
Results: Baseline IgG concentrations were documented in 11 out of the 12 patients prior to ravulizumab treatment (median IgG 9.9 g/L (5-13.5 g/L)). In two female patients a clinically not relevant hypogammaglobulinemia with an associated IgG1 or a combined IgG1/IgG2 deficiency prior to treatment was documented. The data were further stratified with regard to various treatment intervals as multiple analyses were obtained. Throughout observation time IgG concentrations remained within physiologic ranges with no evidence of a treatment-related IgG depletion (median IgG at study endpoint 10.1 g/L (6-13.4 g/L)).
Conclusion: In ravulizumab-treated PNH patients, IgG and IgG subclass levels which are regulated by the FcRn remained unaffected. Therefore, no treatment associated hypogammaglobulinemia is to be feared under chronic ravulizumab therapy.
Conflict of interest statement
A.R. and U.D. receive honoraria, consulting fees, and research support from Alexion Pharmaceuticals, Inc. S.R. is an employee and stockholder of Alexion Pharmaceuticals, Inc. The commercial affiliation of A.R., U.D., and S.R. does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


Similar articles
-
Patient preferences and quality of life implications of ravulizumab (every 8 weeks) and eculizumab (every 2 weeks) for the treatment of paroxysmal nocturnal hemoglobinuria.PLoS One. 2020 Sep 4;15(9):e0237497. doi: 10.1371/journal.pone.0237497. eCollection 2020. PLoS One. 2020. PMID: 32886668 Free PMC article. Clinical Trial.
-
Dosing Patterns of Patients with Paroxysmal Nocturnal Hemoglobinuria Treated with Ravulizumab in the United States: A Retrospective Claims-Based Analysis.Adv Ther. 2024 Jan;41(1):413-430. doi: 10.1007/s12325-023-02725-5. Epub 2023 Nov 24. Adv Ther. 2024. PMID: 37999832 Free PMC article.
-
Phase 3 Study of Subcutaneous Versus Intravenous Ravulizumab in Eculizumab-Experienced Adult Patients with PNH: Primary Analysis and 1-Year Follow-Up.Adv Ther. 2023 Jan;40(1):211-232. doi: 10.1007/s12325-022-02339-3. Epub 2022 Oct 22. Adv Ther. 2023. PMID: 36272026 Free PMC article. Clinical Trial.
-
Pharmacological and clinical profile of ravulizumab 100 mg/mL formulation for paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome.Expert Rev Clin Pharmacol. 2023 May;16(5):401-410. doi: 10.1080/17512433.2023.2209317. Epub 2023 May 9. Expert Rev Clin Pharmacol. 2023. PMID: 37128905 Review.
-
Ravulizumab for the treatment of paroxysmal nocturnal hemoglobinuria.Expert Opin Biol Ther. 2020 Mar;20(3):227-237. doi: 10.1080/14712598.2020.1725468. Epub 2020 Feb 14. Expert Opin Biol Ther. 2020. PMID: 32011183 Review.
Cited by
-
Safety Profile of Monoclonal Antibodies and Subsequent Drug Developments in the Treatment of Paroxysmal Nocturnal Hemoglobinuria.Medicina (Kaunas). 2024 Feb 24;60(3):379. doi: 10.3390/medicina60030379. Medicina (Kaunas). 2024. PMID: 38541105 Free PMC article. Review.
-
Ravulizumab in Myasthenia Gravis: A Review of the Current Evidence.Neuropsychiatr Dis Treat. 2023 Dec 1;19:2639-2655. doi: 10.2147/NDT.S374694. eCollection 2023. Neuropsychiatr Dis Treat. 2023. PMID: 38059203 Free PMC article. Review.
-
Long-term efficacy and safety of ravulizumab in adults with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis: results from the phase 3 CHAMPION MG open-label extension.J Neurol. 2023 Aug;270(8):3862-3875. doi: 10.1007/s00415-023-11699-x. Epub 2023 Apr 27. J Neurol. 2023. PMID: 37103755 Free PMC article. Clinical Trial.
-
Current Opinions on the Clinical Utility of Ravulizumab for the Treatment of Paroxysmal Nocturnal Hemoglobinuria.Ther Clin Risk Manag. 2021 Dec 14;17:1343-1351. doi: 10.2147/TCRM.S273360. eCollection 2021. Ther Clin Risk Manag. 2021. PMID: 34934322 Free PMC article. Review.
-
In Translation: FcRn across the Therapeutic Spectrum.Int J Mol Sci. 2021 Mar 17;22(6):3048. doi: 10.3390/ijms22063048. Int J Mol Sci. 2021. PMID: 33802650 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

