Osthole-loaded N-octyl-O-sulfonyl chitosan micelles (NSC-OST) inhibits RANKL-induced osteoclastogenesis and prevents ovariectomy-induced bone loss in rats
- PMID: 32126148
- PMCID: PMC7171421
- DOI: 10.1111/jcmm.15064
Osthole-loaded N-octyl-O-sulfonyl chitosan micelles (NSC-OST) inhibits RANKL-induced osteoclastogenesis and prevents ovariectomy-induced bone loss in rats
Abstract
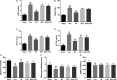
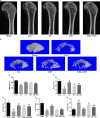
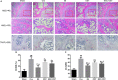
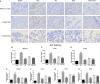
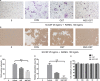
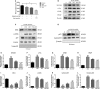
Osthole (OST), a derivative of Fructus Cnidii, has been proved to have potential anti-osteoporosis effects in our recent studies. However, its pharmacological effects are limited in the human body because of poor solubility and bioavailability. Under the guidance of the classical theory of Chinese medicine, Osthole-loaded N-octyl-O-sulfonyl chitosan micelles (NSC-OST), which has not previously been reported in the literature, was synthesized in order to overcome the defects and obtain better efficacy. In this study, we found that NSC-OST inhibited on the formation and resorption activity of osteoclasts through using a bone marrow macrophage (BMM)-derived osteoclast culture system in vitro, rather than affecting the viability of cells. We also found that NSC-OST inhibited osteoclast formation, hydroxyapatite resorption and RANKL-induced osteoclast marker protein expression. In terms of mechanism, NSC-OST suppressed the NFATc1 transcriptional activity and the activation of NF-κB signalling pathway. In vivo, ovariectomized (OVX) rat models were established for further research. We found that NSC-OST can attenuate bone loss in OVX rats through inhibiting osteoclastogenesis. Consistent with our hypothesis, NSC-OST is more effective than OST in parts of the results. Taken together, our findings suggest that NSC-OST can suppress RANKL-induced osteoclastogenesis and prevents ovariectomy-induced bone loss in rats and could be considered a safe and more effective anti-osteoporosis drug than OST.
Keywords: NFATc1; NSC-OST; homology of medicine and food; mutual promotion; osteoclasts; osteoporosis.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
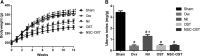
Similar articles
-
Glaucocalyxin A suppresses osteoclastogenesis induced by RANKL and osteoporosis induced by ovariectomy by inhibiting the NF-κB and Akt pathways.J Ethnopharmacol. 2021 Aug 10;276:114176. doi: 10.1016/j.jep.2021.114176. Epub 2021 Apr 30. J Ethnopharmacol. 2021. PMID: 33933570
-
Tectorigenin inhibits RANKL-induced osteoclastogenesis via suppression of NF-κB signalling and decreases bone loss in ovariectomized C57BL/6.J Cell Mol Med. 2018 Oct;22(10):5121-5131. doi: 10.1111/jcmm.13801. Epub 2018 Jul 31. J Cell Mol Med. 2018. PMID: 30063119 Free PMC article.
-
Osthole inhibits osteoclasts formation and bone resorption by regulating NF-κB signaling and NFATc1 activations stimulated by RANKL.J Cell Biochem. 2019 Sep;120(9):16052-16061. doi: 10.1002/jcb.28886. Epub 2019 May 13. J Cell Biochem. 2019. PMID: 31081953
-
Cnidii Fructus: A traditional Chinese medicine herb and source of antiosteoporotic drugs.Phytomedicine. 2024 Jun;128:155375. doi: 10.1016/j.phymed.2024.155375. Epub 2024 Jan 18. Phytomedicine. 2024. PMID: 38507853 Review.
-
IL-33/IL-31 Axis in Osteoporosis.Int J Mol Sci. 2020 Feb 13;21(4):1239. doi: 10.3390/ijms21041239. Int J Mol Sci. 2020. PMID: 32069819 Free PMC article. Review.
Cited by
-
Effects of osthole on osteoporotic rats: a systematic review and meta-analysis.Pharm Biol. 2022 Dec;60(1):1625-1634. doi: 10.1080/13880209.2022.2110267. Pharm Biol. 2022. PMID: 35980123 Free PMC article.
-
Herbal medicine derived carbon dots: synthesis and applications in therapeutics, bioimaging and sensing.J Nanobiotechnology. 2021 Oct 13;19(1):320. doi: 10.1186/s12951-021-01072-3. J Nanobiotechnology. 2021. PMID: 34645456 Free PMC article. Review.
-
lncTIMP3 promotes osteogenic differentiation of bone marrow mesenchymal stem cells via miR-214/Smad4 axis to relieve postmenopausal osteoporosis.Mol Biol Rep. 2024 Jun 1;51(1):719. doi: 10.1007/s11033-024-09652-w. Mol Biol Rep. 2024. PMID: 38824271
-
Role of αVβ3 in Prostate Cancer: Metastasis Initiator and Important Therapeutic Target.Onco Targets Ther. 2020 Jul 28;13:7411-7422. doi: 10.2147/OTT.S258252. eCollection 2020. Onco Targets Ther. 2020. PMID: 32801764 Free PMC article. Review.
-
Quercetin Attenuates Osteoporosis in Orchiectomy Mice by Regulating Glucose and Lipid Metabolism via the GPRC6A/AMPK/mTOR Signaling Pathway.Front Endocrinol (Lausanne). 2022 Apr 25;13:849544. doi: 10.3389/fendo.2022.849544. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35547008 Free PMC article.
References
-
- Lagari VS, Levis S. Phytoestrogens in the prevention of postmenopausal bone loss. J Clin Densitom. 2013;16(4):445‐449. - PubMed
-
- D'Amelio P, Grimaldi A, Di Bella S, et al. Estrogen deficiency increases osteoclastogenesis up‐regulating T cells activity: a key mechanism in osteoporosis. Bone. 2008;43(1):92‐100. - PubMed
-
- Bar‐Shavit Z. The osteoclast: a multinucleated, hematopoietic‐origin, bone‐resorbing osteoimmune cell. J Cell Biochem. 2007;102(5):1130‐1139. - PubMed
-
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337‐342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

