Cisplatin resistance in gastric cancer cells is involved with GPR30-mediated epithelial-mesenchymal transition
- PMID: 32052561
- PMCID: PMC7131920
- DOI: 10.1111/jcmm.15055
Cisplatin resistance in gastric cancer cells is involved with GPR30-mediated epithelial-mesenchymal transition
Abstract
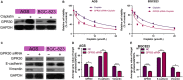
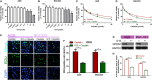
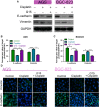
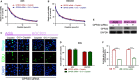
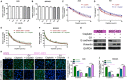
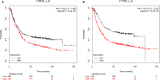
Cisplatin is the major chemotherapeutic drug in gastric cancer, particularly in treating advanced gastric cancer. Tumour cells often develop resistance to chemotherapeutic drugs, which seriously affects the efficacy of chemotherapy. GPR30 is a novel oestrogen receptor that is involved in the invasion, metastasis and drug resistance of many tumours. Targeting GPR30 has been shown to increase the drug sensitivity of breast cancer cells. However, few studies have investigated the role of GPR30 in gastric cancer. Epithelial-mesenchymal transition (EMT) has been shown to be associated with the development of chemotherapeutic drug resistance. In this study, we demonstrated that GPR30 is involved in cisplatin resistance by promoting EMT in gastric cancer. GPR30 knockdown resulted in increased sensitivity of different gastric cancer (GC) cells to cisplatin and alterations in the epithelial/mesenchymal markers. Furthermore, G15 significantly enhanced the cisplatin sensitivity of GC cells while G1 inhibited this phenomenon. In addition, EMT occurred when AGS and BGC-823 were treated with cisplatin. Down-regulation of GPR30 with G15 inhibited this transformation, while G1 promoted it. Taken together, these results revealed the role of GPR30 in the formation of cisplatin resistance, suggesting that targeting GPR30 signalling may be a potential strategy for improving the efficacy of chemotherapy in gastric cancer.
Keywords: G1; G15; GPR30; cisplatin; epithelial-mesenchymal transition (EMT); gastric cancer; resistance.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Upregulation of FAM3B Promotes Cisplatin Resistance in Gastric Cancer by Inducing Epithelial-Mesenchymal Transition.Med Sci Monit. 2020 May 22;26:e921002. doi: 10.12659/MSM.921002. Med Sci Monit. 2020. PMID: 32442162 Free PMC article.
-
MicroRNA-574-3p regulates epithelial mesenchymal transition and cisplatin resistance via targeting ZEB1 in human gastric carcinoma cells.Gene. 2019 Jun 5;700:110-119. doi: 10.1016/j.gene.2019.03.043. Epub 2019 Mar 24. Gene. 2019. PMID: 30917930
-
Cisplatin resistance in gastric cancer cells is associated with HER2 upregulation-induced epithelial-mesenchymal transition.Sci Rep. 2016 Feb 5;6:20502. doi: 10.1038/srep20502. Sci Rep. 2016. PMID: 26846307 Free PMC article.
-
Association of the Epithelial-Mesenchymal Transition (EMT) with Cisplatin Resistance.Int J Mol Sci. 2020 Jun 3;21(11):4002. doi: 10.3390/ijms21114002. Int J Mol Sci. 2020. PMID: 32503307 Free PMC article. Review.
-
The relationship between platinum drug resistance and epithelial-mesenchymal transition.Arch Toxicol. 2017 Feb;91(2):605-619. doi: 10.1007/s00204-016-1912-7. Epub 2016 Dec 28. Arch Toxicol. 2017. PMID: 28032148 Review.
Cited by
-
Roles of G Protein-Coupled Receptors (GPCRs) in Gastrointestinal Cancers: Focus on Sphingosine 1-Shosphate Receptors, Angiotensin II Receptors, and Estrogen-Related GPCRs.Cells. 2021 Nov 3;10(11):2988. doi: 10.3390/cells10112988. Cells. 2021. PMID: 34831211 Free PMC article. Review.
-
Identification of an EMT-Related Gene Signature for Predicting Overall Survival in Gastric Cancer.Front Genet. 2021 Jun 24;12:661306. doi: 10.3389/fgene.2021.661306. eCollection 2021. Front Genet. 2021. PMID: 34249086 Free PMC article.
-
Inhibition of GPR30 sensitized gefitinib to NSCLC cells via regulation of epithelial-mesenchymal transition.Int J Immunopathol Pharmacol. 2023 Jan-Dec;37:3946320231210737. doi: 10.1177/03946320231210737. Int J Immunopathol Pharmacol. 2023. PMID: 37890097 Free PMC article.
-
Does G Protein-Coupled Estrogen Receptor 1 Contribute to Cisplatin-Induced Acute Kidney Injury in Male Mice?Int J Mol Sci. 2022 Jul 27;23(15):8284. doi: 10.3390/ijms23158284. Int J Mol Sci. 2022. PMID: 35955435 Free PMC article.
-
Tetrahydroxanthene-1,3(2H)-diones and Oxidized Hexadiene Derivatives from Uvaria leptopoda and Their Biological Activities.J Nat Prod. 2024 Jun 28;87(6):1611-1617. doi: 10.1021/acs.jnatprod.4c00248. Epub 2024 May 28. J Nat Prod. 2024. PMID: 38805684 Free PMC article.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7‐30. - PubMed
-
- Shen L, Shan YS, Hu HM, et al. Management of gastric cancer in Asia: resource‐stratified guidelines. Lancet Oncol. 2013;14:e535‐e547. - PubMed
-
- Sasaki K, Onodera S, Otsuka K, et al. Validity of neoadjuvant chemotherapy with docetaxel, cisplatin, and S‐1 for resectable locally advanced gastric cancer. Med Oncol. 2017;34:139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

