Treatment with anti-neonatal Fc receptor (FcRn) antibody ameliorates experimental epidermolysis bullosa acquisita in mice
- PMID: 31975370
- PMCID: PMC7174883
- DOI: 10.1111/bph.14986
Treatment with anti-neonatal Fc receptor (FcRn) antibody ameliorates experimental epidermolysis bullosa acquisita in mice
Abstract
Background and purpose: Pemphigus and pemphigoid diseases are characterized and caused predominantly by IgG autoantibodies targeting structural proteins of the skin. Their current treatment relies on general and prolonged immunosuppression that causes severe adverse events, including death. Hence, novel safe and more effective treatments are urgently needed. Due to its' physiological functions, the neonatal Fc receptor (FcRn) has emerged as a potential therapeutic target for pemphigus and pemphigoid, primarily because IgG is protected from proteolysis after uptake into endothelial cells. Thus, blockade of FcRn would reduce circulating autoantibody concentrations. However, long-term effects of pharmacological FcRn inhibition in therapeutic settings of autoimmune diseases are unknown.
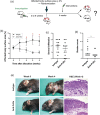
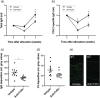
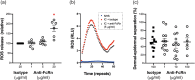
Experimental approach: Therapeutic effects of FcRn blockade were investigated in a murine model of the prototypical autoantibody-mediated pemphigoid disease, epidermolysis bullosa acquisita (EBA). B6.SJL-H2s C3c/1CyJ mice with clinically active disease were randomized to receive either an anti-FcRn monoclonal antibody (4470) or an isotype control over 4 weeks.
Key results: While clinical disease continued to worsen in isotype control-treated mice, overall disease severity continuously decreased in mice injected with 4470, leading to almost complete remission in over 25% of treated mice. These clinical findings were paralleled by a reduction of autoantibody concentrations. Reduction of autoantibody concentrations, rather than modulating neutrophil activation, was responsible for the observed therapeutic effects.
Conclusion and implications: The clinical efficacy of anti-FcRn treatment in this prototypical autoantibody-mediated disease encourages further development of anti-FcRn antibodies for clinical use in pemphigoid diseases and potentially in other autoantibody mediated diseases.
© 2020 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
In the last 3 years, R.J.L. has received research funding from Almirall, True North Therapeutics, UCB Pharma, ArgenX, TxCell, Topadur, Incyte, and Admirx and fees for consulting or speaking from ArgenX, Immunogenetics, Novartis, and Lilly. E.S. has received research funding from Almirall, UCB Pharma, ArgenX, TxCell, Incyte, Novartis, Euroimmun, and Admirx and fees for consulting or speaking from ArgenX, UCB, TxCell, Novartis, Fresenius, and Almirall. A.S. is an employee of UCB, and P.W. and B.S. are former employees of UCB. All other authors declare no conflict of interest.
Figures

Similar articles
-
Neonatal Fc receptor deficiency protects from tissue injury in experimental epidermolysis bullosa acquisita.J Mol Med (Berl). 2008 Aug;86(8):951-9. doi: 10.1007/s00109-008-0366-7. Epub 2008 Jun 10. J Mol Med (Berl). 2008. PMID: 18542899
-
Fcγ Receptor IIB Controls Skin Inflammation in an Active Model of Epidermolysis Bullosa Acquisita.Front Immunol. 2020 Jan 14;10:3012. doi: 10.3389/fimmu.2019.03012. eCollection 2019. Front Immunol. 2020. PMID: 31993051 Free PMC article.
-
Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases.J Clin Invest. 2005 Dec;115(12):3440-50. doi: 10.1172/JCI24394. Epub 2005 Nov 10. J Clin Invest. 2005. PMID: 16284651 Free PMC article.
-
Neonatal Fc receptor in human immunity: Function and role in therapeutic intervention.J Allergy Clin Immunol. 2020 Sep;146(3):467-478. doi: 10.1016/j.jaci.2020.07.015. J Allergy Clin Immunol. 2020. PMID: 32896307 Review.
-
Immune mechanism-targeted treatment of experimental epidermolysis bullosa acquisita.Expert Rev Clin Immunol. 2015;11(12):1365-78. doi: 10.1586/1744666X.2015.1085801. Epub 2015 Oct 15. Expert Rev Clin Immunol. 2015. PMID: 26471717 Review.
Cited by
-
Epidermolysis Bullosa Acquisita-Current and Emerging Treatments.J Clin Med. 2023 Feb 1;12(3):1139. doi: 10.3390/jcm12031139. J Clin Med. 2023. PMID: 36769788 Free PMC article. Review.
-
FcRn inhibitors: a novel option for the treatment of myasthenia gravis.Neural Regen Res. 2023 Aug;18(8):1637-1644. doi: 10.4103/1673-5374.363824. Neural Regen Res. 2023. PMID: 36751773 Free PMC article. Review.
-
The therapeutic age of the neonatal Fc receptor.Nat Rev Immunol. 2023 Jul;23(7):415-432. doi: 10.1038/s41577-022-00821-1. Epub 2023 Feb 1. Nat Rev Immunol. 2023. PMID: 36726033 Free PMC article. Review.
-
Therapeutic effects of Fc gamma RIV inhibition are mediated by selectively blocking immune complex-induced neutrophil activation in epidermolysis bullosa acquisita.Front Immunol. 2022 Oct 13;13:938306. doi: 10.3389/fimmu.2022.938306. eCollection 2022. Front Immunol. 2022. PMID: 36311755 Free PMC article.
-
Antineonatal Fc Receptor Antibody Treatment Ameliorates MOG-IgG-Associated Experimental Autoimmune Encephalomyelitis.Neurol Neuroimmunol Neuroinflamm. 2022 Jan 13;9(2):e1134. doi: 10.1212/NXI.0000000000001134. Print 2022 Mar. Neurol Neuroimmunol Neuroinflamm. 2022. PMID: 35027475 Free PMC article.
References
-
- Alexander, S. P. , Roberts, R. E. , Broughton, B. R. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Insel, P. A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology . British Journal of Pharmacology, 175(3), 407–411. - PMC - PubMed
-
- Amagai, M. , Ikeda, S. , Shimizu, H. , Iizuka, H. , Hanada, K. , Aiba, S. , … Takigawa, M. (2009). A randomized double‐blind trial of intravenous immunoglobulin for pemphigus. Journal of the American Academy of Dermatology, 60(4), 595–603. - PubMed
-
- Amber, K. T. , Murrell, D. F. , Schmidt, E. , Joly, P. , & Borradori, L. (2018). Autoimmune subepidermal bullous diseases of the skin and mucosae: Clinical features, diagnosis, and management. Clinical Reviews in Allergy and Immunology, 54, 26–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

