Three-Year Outcomes of a Randomized, Double-Blind, Placebo-Controlled Study Assessing Safety and Efficacy of C1 Esterase Inhibitor for Prevention of Delayed Graft Function in Deceased Donor Kidney Transplant Recipients
- PMID: 31843975
- PMCID: PMC6946080
- DOI: 10.2215/CJN.04840419
Three-Year Outcomes of a Randomized, Double-Blind, Placebo-Controlled Study Assessing Safety and Efficacy of C1 Esterase Inhibitor for Prevention of Delayed Graft Function in Deceased Donor Kidney Transplant Recipients
Abstract
Background and objectives: Delayed graft function is related to ischemia-reperfusion injury and may be complement dependent. We previously reported from a randomized, placebo-controlled trial that treatment with C1 esterase inhibitor was associated with a shorter duration of delayed graft function and higher eGFR at 1 year. Here, we report longer-term outcomes from this trial.
Design, setting, participants, & measurements: This is a post hoc analysis of a phase 1/2, randomized, controlled trial enrolling 70 recipients of deceased donor kidney transplants at risk for delayed graft function (NCT02134314). Subjects were randomized to receive C1 esterase inhibitor 50 U/kg (n=35) or placebo (n=35) intraoperatively and at 24 hours. The cumulative incidence functions method was used to compare graft failure and death over 3.5 years. eGFR slopes were compared using a linear mixed effects model.
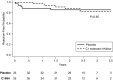
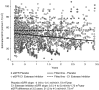
Results: Three deaths occurred among C1 esterase inhibitor-treated patients compared with none receiving placebo. Seven graft failures developed in the placebo group compared with one among C1 esterase inhibitor-treated recipients; the cumulative incidence of graft failure was lower over 3.5 years among C1 esterase inhibitor-treated recipients compared with placebo (P=0.03). Although no difference in eGFR slopes was observed between groups (P for group-time interaction =0.12), eGFR declined in placebo-treated recipients (-4 ml/min per 1.73 m2 per year; 95% confidence interval, -8 to -0.1) but was stable in C1 esterase inhibitor-treated patients (eGFR slope: 0.5 ml/min per 1.73 m2 per year; 95% confidence interval, -4 to 5). At 3.5 years, eGFR was 56 ml/min per 1.73 m2 (95% confidence interval, 42 to 70) in the C1 esterase inhibitor group versus 35 ml/min per 1.73 m2 (95% confidence interval, 21 to 48) in the placebo group, with an estimated mean eGFR difference of 21 ml/min per 1.73 m2 (95% confidence interval, 2 to 41 ml/min per 1.73 m2).
Conclusions: Treatment of patients at risk for ischemia-reperfusion injury and delayed graft function with C1 esterase inhibitor was associated with a lower incidence of graft failure.
Keywords: complement C1 inhibitor protein; complement c1s; death; delayed graft function; double-blind method; epidermal growth factor receptor; glomerular filtration rate; human EGFR protein; humans; incidence; ischemia-reperfusion; kidney transplantation; reperfusion injury; tissue donors.
Copyright © 2020 by the American Society of Nephrology.
Figures


Comment in
-
Medical Therapies to Reduce Delayed Graft Function and Improve Long-Term Graft Survival: Are We Making Progress?Clin J Am Soc Nephrol. 2020 Jan 7;15(1):13-15. doi: 10.2215/CJN.13961119. Epub 2019 Dec 16. Clin J Am Soc Nephrol. 2020. PMID: 31911413 Free PMC article. No abstract available.
Similar articles
-
A phase I/II, double-blind, placebo-controlled study assessing safety and efficacy of C1 esterase inhibitor for prevention of delayed graft function in deceased donor kidney transplant recipients.Am J Transplant. 2018 Dec;18(12):2955-2964. doi: 10.1111/ajt.14767. Epub 2018 May 14. Am J Transplant. 2018. PMID: 29637714 Clinical Trial.
-
A phase I/II placebo-controlled trial of C1-inhibitor for prevention of antibody-mediated rejection in HLA sensitized patients.Transplantation. 2015 Feb;99(2):299-308. doi: 10.1097/TP.0000000000000592. Transplantation. 2015. PMID: 25606785 Clinical Trial.
-
Peri-transplant aminophylline in pediatric kidney transplant recipients of donation after brain death: a double-blinded placebo-controlled randomized clinical trial.Pediatr Nephrol. 2020 Sep;35(9):1729-1736. doi: 10.1007/s00467-020-04561-z. Epub 2020 May 16. Pediatr Nephrol. 2020. PMID: 32418145 Clinical Trial.
-
Update on C1 Esterase Inhibitor in Human Solid Organ Transplantation.Transplantation. 2019 Sep;103(9):1763-1775. doi: 10.1097/TP.0000000000002717. Transplantation. 2019. PMID: 30946220 Review.
-
Ischaemia reperfusion injury: mechanisms of progression to chronic graft dysfunction.Pediatr Nephrol. 2019 Jun;34(6):951-963. doi: 10.1007/s00467-018-3940-4. Epub 2018 Mar 30. Pediatr Nephrol. 2019. PMID: 29603016 Free PMC article. Review.
Cited by
-
Antibody-mediated rejection in xenotransplantation: Can it be prevented or reversed?Xenotransplantation. 2023 Jul-Aug;30(4):e12816. doi: 10.1111/xen.12816. Epub 2023 Aug 7. Xenotransplantation. 2023. PMID: 37548030 Free PMC article. Review.
-
SOX9 switch links regeneration to fibrosis at the single-cell level in mammalian kidneys.Science. 2024 Feb 23;383(6685):eadd6371. doi: 10.1126/science.add6371. Epub 2024 Feb 23. Science. 2024. PMID: 38386758 Free PMC article.
-
Approaches for Controlling Antibody-Mediated Allograft Rejection Through Targeting B Cells.Front Immunol. 2021 Jul 1;12:682334. doi: 10.3389/fimmu.2021.682334. eCollection 2021. Front Immunol. 2021. PMID: 34276669 Free PMC article. Review.
-
Nanobodies: new avenue to treat kidney disease.Cell Tissue Res. 2021 Aug;385(2):445-456. doi: 10.1007/s00441-021-03479-8. Epub 2021 Jun 16. Cell Tissue Res. 2021. PMID: 34131806 Free PMC article. Review.
-
Role of Complement System in Kidney Transplantation: Stepping From Animal Models to Clinical Application.Front Immunol. 2022 Feb 25;13:811696. doi: 10.3389/fimmu.2022.811696. eCollection 2022. Front Immunol. 2022. PMID: 35281019 Free PMC article. Review.
References
-
- United States Renal Data System : 2015 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2015
-
- Wu WK, Famure O, Li Y, Kim SJ: Delayed graft function and the risk of acute rejection in the modern era of kidney transplantation. Kidney Int 88: 851–858, 2015 - PubMed
-
- Lim WH, Johnson DW, Teixeira-Pinto A, Wong G: Association between duration of delayed graft function, acute rejection, and allograft outcome after deceased donor kidney transplantation. Transplantation 103: 412–419, 2019 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

