Phase 2 study of efgartigimod, a novel FcRn antagonist, in adult patients with primary immune thrombocytopenia
- PMID: 31821591
- PMCID: PMC7004056
- DOI: 10.1002/ajh.25680
Phase 2 study of efgartigimod, a novel FcRn antagonist, in adult patients with primary immune thrombocytopenia
Abstract
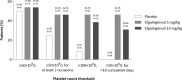
Primary immune thrombocytopenia (ITP) is an acquired autoimmune bleeding disorder, characterized by a low platelet count (<100 × 109 /L) in the absence of other causes associated with thrombocytopenia. In most patients, IgG autoantibodies directed against platelet receptors can be detected. They accelerate platelet clearance and destruction, inhibit platelet production, and impair platelet function, resulting in increased risk of bleeding and impaired quality of life. Efgartigimod is a human IgG1 antibody Fc-fragment, a natural ligand of the neonatal Fc receptor (FcRn), engineered for increased affinity to FcRn, while preserving its characteristic pH-dependent binding. Efgartigimod blocks FcRn, preventing IgG recycling, and causing targeted IgG degradation. In this Phase 2 study, 38 patients were randomized 1:1:1 to receive four weekly intravenous infusions of either placebo (N = 12) or efgartigimod at a dose of 5 mg/kg (N = 13) or 10 mg/kg (N = 13). This short treatment cycle of efgartigimod in patients with ITP, predominantly refractory to previous lines of therapy, was shown to be well tolerated, and demonstrated a favorable safety profile consistent with Phase 1 data. Efgartigimod induced a rapid reduction of total IgG levels (up to 63.7% mean change from baseline), which was associated with clinically relevant increases in platelet counts (46% patients on efgartigimod vs 25% on placebo achieved a platelet count of ≥50 × 109 /L on at least two occasions, and 38% vs 0% achieved ≥50 × 109 /L for at least 10 cumulative days), and a reduced proportion of patients with bleeding. Taken together, these data warrant further evaluation of FcRn antagonism as a novel therapeutic approach in ITP.
© 2019 The Authors. American Journal of Hematology published by Wiley Periodicals, Inc.
Conflict of interest statement
Adrian C. Newland: consultant for Amgen, Angle, argenx, Dova, Novartis, Ono, Rigel, and Shionogi; received funding from Amgen, Novartis, and Rigel; received honoraria directly from Amgen, Angle, argenx, Dova, Novartis, Ono, Rigel, and Shionogi; and paid expert testimony from argenx and Rigel. Blanca Sánchez‐González: received honoraria directly and paid expert testimony from Novartis, Takeda, Amgen, Alexion, Gilead and Shire; and Board of Directors or its advisory committees at Novartis, Takeda, Amgen. László Rejtő, Miklos Egyed, and Nataliya Romanyuk: none. E. Sally Ward: receives funding and royalty payments from argenx, and has equity ownership in argenx. Marc Michel: consultant for Novartis, Amgen, and Rigel. Howard A. Liebman: consultant for argenx, Novartis, Rigel, Pfizer, Dova, and received funding from Janssen and Bristol‐Myers. David J. Kuter: consultant for Actelion (Syntimmune), Agios, Alnylam, Amgen, argenx, Bristol Myers Squibb (BMS), Caremark, Daiichi Sankyo, Dova, Kyowa‐Kirin, Merck Sharp Dohme, Momenta, Novartis, Pfizer, Platelet Disorder Support Association, Principia, Protalex, Protalix, Rigel, Sanofi, Genzyme, Shionogi, Shire, Takeda (Bioverativ), UCB, Up‐To‐Date, Zafgen; and received funding from Actelion (Syntimmune), Agios, Alnylam, Amgen, argenx, Bristol Myers Squibb (BMS), Kezar, Principia, Protalex, Rigel, and Takeda (Bioverativ). Katrien Verschueren: consultant for argenx. Marie Godar, Domenica Gandini, Peter Ulrichts, Jon Beauchamp, Torsten Dreier, Hans de Haard, and Nicolas Leupin: employees and equity ownership in argenx.
Figures

Similar articles
-
Efficacy and safety of the neonatal Fc receptor inhibitor efgartigimod in adults with primary immune thrombocytopenia (ADVANCE IV): a multicentre, randomised, placebo-controlled, phase 3 trial.Lancet. 2023 Nov 4;402(10413):1648-1659. doi: 10.1016/S0140-6736(23)01460-5. Epub 2023 Sep 28. Lancet. 2023. PMID: 37778358 Clinical Trial.
-
Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans.J Clin Invest. 2018 Oct 1;128(10):4372-4386. doi: 10.1172/JCI97911. Epub 2018 Jul 24. J Clin Invest. 2018. PMID: 30040076 Free PMC article. Clinical Trial.
-
Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis.Neurology. 2019 Jun 4;92(23):e2661-e2673. doi: 10.1212/WNL.0000000000007600. Epub 2019 May 22. Neurology. 2019. PMID: 31118245 Free PMC article. Clinical Trial.
-
Efgartigimod alfa for the treatment of primary immune thrombocytopenia.Ther Adv Hematol. 2023 May 10;14:20406207231172831. doi: 10.1177/20406207231172831. eCollection 2023. Ther Adv Hematol. 2023. PMID: 37188068 Free PMC article. Review.
-
Clinical efficacy and safety of efgartigimod for treatment of myasthenia gravis.Immunotherapy. 2023 Jun;15(8):553-563. doi: 10.2217/imt-2022-0298. Epub 2023 Apr 4. Immunotherapy. 2023. PMID: 37013835 Review.
Cited by
-
Food and Drug Administration (FDA) Approvals of Biological Drugs in 2023.Biomedicines. 2024 Sep 2;12(9):1992. doi: 10.3390/biomedicines12091992. Biomedicines. 2024. PMID: 39335511 Free PMC article. Review.
-
2025 update on clinical trials in immune thrombocytopenia.Am J Hematol. 2024 Nov;99(11):2178-2190. doi: 10.1002/ajh.27448. Epub 2024 Aug 6. Am J Hematol. 2024. PMID: 39105413 Review.
-
Efgartigimod as a novel FcRn inhibitor for autoimmune disease.Neurol Sci. 2024 Sep;45(9):4229-4241. doi: 10.1007/s10072-024-07460-5. Epub 2024 Apr 22. Neurol Sci. 2024. PMID: 38644454 Review.
-
The 2022 review of the 2019 American Society of Hematology guidelines on immune thrombocytopenia.Blood Adv. 2024 Jul 9;8(13):3578-3582. doi: 10.1182/bloodadvances.2023012541. Blood Adv. 2024. PMID: 38608258 Free PMC article. Review.
-
Efgartigimod in the treatment of Guillain-Barré syndrome.J Neurol. 2024 Jun;271(6):3506-3511. doi: 10.1007/s00415-024-12321-4. Epub 2024 Mar 26. J Neurol. 2024. PMID: 38532142
References
-
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386‐2393. - PubMed
-
- Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Crowther MA. The American Society of Hematology 2011 evidence‐based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190‐4207. - PubMed
-
- Zufferey A, Kapur R, Semple J. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med. 2017;6(2):E16. - PubMed
-
- Cohen YC, Djulbegovic B, Shamai‐Lubovitz O, Mozes B. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch Intern Med. 2000;160(11):1630‐1638. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
