Amyloid-Beta (Aβ) Plaques Promote Seeding and Spreading of Alpha-Synuclein and Tau in a Mouse Model of Lewy Body Disorders with Aβ Pathology
- PMID: 31759806
- PMCID: PMC6981053
- DOI: 10.1016/j.neuron.2019.10.010
Amyloid-Beta (Aβ) Plaques Promote Seeding and Spreading of Alpha-Synuclein and Tau in a Mouse Model of Lewy Body Disorders with Aβ Pathology
Erratum in
-
Amyloid-Beta (Aβ) Plaques Promote Seeding and Spreading of Alpha-Synuclein and Tau in a Mouse Model of Lewy Body Disorders with Aβ Pathology.Neuron. 2023 Nov 15;111(22):3699. doi: 10.1016/j.neuron.2023.10.030. Neuron. 2023. PMID: 37972565 Free PMC article. No abstract available.
Abstract
Studies have shown an overlap of Aβ plaques, tau tangles, and α-synuclein (α-syn) pathologies in the brains of Alzheimer's disease (AD) and Parkinson's disease (PD) with dementia (PDD) patients, with increased pathological burden correlating with severity of cognitive and motor symptoms. Despite the observed co-pathology and concomitance of motor and cognitive phenotypes, the consequences of the primary amyloidogenic protein on the secondary pathologies remain poorly understood. To better define the relationship between α-syn and Aβ plaques, we injected α-syn preformed fibrils (α-syn mpffs) into mice with abundant Aβ plaques. Aβ deposits dramatically accelerated α-syn pathogenesis and spread throughout the brain. Remarkably, hyperphosphorylated tau (p-tau) was induced in α-syn mpff-injected 5xFAD mice. Finally, α-syn mpff-injected 5xFAD mice showed neuron loss that correlated with the progressive decline of cognitive and motor performance. Our findings suggest a "feed-forward" mechanism whereby Aβ plaques enhance endogenous α-syn seeding and spreading over time post-injection with mpffs.
Keywords: Lewy bodies; Parkinson’s disease; alpha-synuclein; alzheimer's disease; amyloid-beta plaques; comorbidity; dementia; parkinsonism; tau.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interest.
Figures


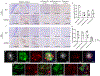
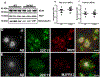




Comment in
-
Aβ Puts the Alpha in Synuclein.Neuron. 2020 Jan 22;105(2):205-206. doi: 10.1016/j.neuron.2020.01.001. Neuron. 2020. PMID: 31972141
Similar articles
-
Significant Overlap of α-Synuclein, Amyloid-β, and Phospho-Tau Pathologies in Neuropathological Diagnosis of Lewy-related Pathology: Evidence from China Human Brain Bank.J Alzheimers Dis. 2021;80(1):447-458. doi: 10.3233/JAD-201548. J Alzheimers Dis. 2021. PMID: 33554920
-
Regional Multiple Pathology Scores Are Associated with Cognitive Decline in Lewy Body Dementias.Brain Pathol. 2015 Jul;25(4):401-8. doi: 10.1111/bpa.12182. Epub 2014 Oct 30. Brain Pathol. 2015. PMID: 25103200 Free PMC article.
-
Human amyloid beta and α-synuclein co-expression in neurons impair behavior and recapitulate features for Lewy body dementia in Caenorhabditis elegans.Biochim Biophys Acta Mol Basis Dis. 2021 Oct 1;1867(10):166203. doi: 10.1016/j.bbadis.2021.166203. Epub 2021 Jun 17. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 34146705
-
Associations Between APOE Variants, Tau and α-Synuclein.Adv Exp Med Biol. 2019;1184:177-186. doi: 10.1007/978-981-32-9358-8_15. Adv Exp Med Biol. 2019. PMID: 32096038 Review.
-
Amyloid β, Tau, and α-Synuclein aggregates in the pathogenesis, prognosis, and therapeutics for neurodegenerative diseases.Prog Neurobiol. 2022 Jul;214:102270. doi: 10.1016/j.pneurobio.2022.102270. Epub 2022 Apr 18. Prog Neurobiol. 2022. PMID: 35447272 Review.
Cited by
-
Longitudinal predictive modeling of tau progression along the structural connectome.Neuroimage. 2021 Aug 15;237:118126. doi: 10.1016/j.neuroimage.2021.118126. Epub 2021 May 4. Neuroimage. 2021. PMID: 33957234 Free PMC article.
-
Intracranial alternating current stimulation facilitates neurogenesis in a mouse model of Alzheimer's disease.Alzheimers Res Ther. 2020 Jul 23;12(1):89. doi: 10.1186/s13195-020-00656-9. Alzheimers Res Ther. 2020. PMID: 32703308 Free PMC article.
-
A postmortem study suggests a revision of the dual-hit hypothesis of Parkinson's disease.NPJ Parkinsons Dis. 2022 Nov 30;8(1):166. doi: 10.1038/s41531-022-00436-2. NPJ Parkinsons Dis. 2022. PMID: 36450732 Free PMC article.
-
Investigating key factors underlying neurodegeneration linked to alpha-synuclein spread.Neuropathol Appl Neurobiol. 2022 Oct;48(6):e12829. doi: 10.1111/nan.12829. Epub 2022 Jul 4. Neuropathol Appl Neurobiol. 2022. PMID: 35727707 Free PMC article.
-
α-synuclein-assisted oligomerization of β-amyloid (1-42).Arch Biochem Biophys. 2022 Mar 15;717:109120. doi: 10.1016/j.abb.2022.109120. Epub 2022 Jan 15. Arch Biochem Biophys. 2022. PMID: 35041853 Free PMC article.
References
-
- Alberdi E, Wyssenbach A, Alberdi M, Sanchez-Gomez MV, Cavaliere F, Rodriguez JJ, Verkhratsky A, and Matute C (2013). Ca(2+) -dependent endoplasmic reticulum stress correlates with astrogliosis in oligomeric amyloid beta-treated astrocytes and in a model of Alzheimer’s disease. Aging cell 12, 292–302. - PubMed
-
- Bachhuber T, Katzmarski N, McCarter JF, Loreth D, Tahirovic S, Kamp F, Abou-Ajram C, Nuscher B, Serrano-Pozo A, Muller A, et al. (2015). Inhibition of amyloid-beta plaque formation by alpha-synuclein. Nature medicine 21, 802–807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

