PR55α regulatory subunit of PP2A inhibits the MOB1/LATS cascade and activates YAP in pancreatic cancer cells
- PMID: 31659153
- PMCID: PMC6817822
- DOI: 10.1038/s41389-019-0172-9
PR55α regulatory subunit of PP2A inhibits the MOB1/LATS cascade and activates YAP in pancreatic cancer cells
Abstract
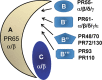
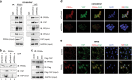
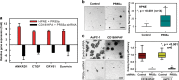
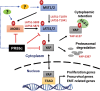
PP2A holoenzyme complexes are responsible for the majority of Ser/Thr phosphatase activities in human cells. Each PP2A consists of a catalytic subunit (C), a scaffold subunit (A), and a regulatory subunit (B). While the A and C subunits each exists only in two highly conserved isoforms, a large number of B subunits share no homology, which determines PP2A substrate specificity and cellular localization. It is anticipated that different PP2A holoenzymes play distinct roles in cellular signaling networks, whereas PP2A has only generally been defined as a putative tumor suppressor, which is mostly based on the loss-of-function studies using pharmacological or biological inhibitors for the highly conserved A or C subunit of PP2A. Recent studies of specific pathways indicate that some PP2A complexes also possess tumor-promoting functions. We have previously reported an essential role of PR55α, a PP2A regulatory subunit, in the support of oncogenic phenotypes, including in vivo tumorigenicity/metastasis of pancreatic cancer cells. In this report, we have elucidated a novel role of PR55α-regulated PP2A in the activation of YAP oncoprotein, whose function is required for anchorage-independent growth during oncogenesis of solid tumors. Our data show two lines of YAP regulation by PR55α: (1) PR55α inhibits the MOB1-triggered autoactivation of LATS1/2 kinases, the core member of the Hippo pathway that inhibits YAP by inducing its proteasomal degradation and cytoplasmic retention and (2) PR55α directly interacts with and regulates YAP itself. Accordingly, PR55α is essential for YAP-promoted gene transcriptions, as well as for anchorage-independent growth, in which YAP plays a key role. In summary, current findings demonstrate a novel YAP activation mechanism based on the PR55α-regulated PP2A phosphatase.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
PR55α Subunit of Protein Phosphatase 2A Supports the Tumorigenic and Metastatic Potential of Pancreatic Cancer Cells by Sustaining Hyperactive Oncogenic Signaling.Cancer Res. 2016 Apr 15;76(8):2243-2253. doi: 10.1158/0008-5472.CAN-15-2119. Epub 2016 Feb 18. Cancer Res. 2016. PMID: 26893480 Free PMC article.
-
p53/FBXL20 axis negatively regulates the protein stability of PR55α, a regulatory subunit of PP2A Ser/Thr phosphatase.Neoplasia. 2021 Dec;23(12):1192-1203. doi: 10.1016/j.neo.2021.10.002. Epub 2021 Oct 30. Neoplasia. 2021. PMID: 34731788 Free PMC article.
-
PR55α-controlled protein phosphatase 2A inhibits p16 expression and blocks cellular senescence induction by γ-irradiation.Aging (Albany NY). 2024 Mar 4;16(5):4116-4137. doi: 10.18632/aging.205619. Epub 2024 Mar 4. Aging (Albany NY). 2024. PMID: 38441530 Free PMC article.
-
[Targeting of PP2A enzymes by viral proteins and cancer signalling].Med Sci (Paris). 2011 Dec;27(12):1106-11. doi: 10.1051/medsci/20112712017. Epub 2011 Dec 23. Med Sci (Paris). 2011. PMID: 22192750 Review. French.
-
PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail).Trends Biochem Sci. 2008 Mar;33(3):113-21. doi: 10.1016/j.tibs.2007.12.004. Trends Biochem Sci. 2008. PMID: 18291659 Review.
Cited by
-
Lysine demethylase 2 (KDM2B) regulates hippo pathway via MOB1 to promote pancreatic ductal adenocarcinoma (PDAC) progression.J Exp Clin Cancer Res. 2020 Jan 15;39(1):13. doi: 10.1186/s13046-019-1489-0. J Exp Clin Cancer Res. 2020. PMID: 31941533 Free PMC article.
-
Control of tissue homeostasis, tumorigenesis, and degeneration by coupled bidirectional bistable switches.PLoS Comput Biol. 2021 Nov 19;17(11):e1009606. doi: 10.1371/journal.pcbi.1009606. eCollection 2021 Nov. PLoS Comput Biol. 2021. PMID: 34797839 Free PMC article.
-
Advancements of anticancer agents by targeting the Hippo signalling pathway: biological activity, selectivity, docking analysis, and structure-activity relationship.Mol Divers. 2024 Oct 22. doi: 10.1007/s11030-024-11009-1. Online ahead of print. Mol Divers. 2024. PMID: 39436581 Review.
-
PP2A-B55: substrates and regulators in the control of cellular functions.Oncogene. 2022 Jan;41(1):1-14. doi: 10.1038/s41388-021-02068-x. Epub 2021 Oct 22. Oncogene. 2022. PMID: 34686773 Review.
-
Programmed cell death 10 promotes metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma via PP2Ac-mediated YAP activation.Cell Death Dis. 2021 Sep 14;12(9):849. doi: 10.1038/s41419-021-04139-z. Cell Death Dis. 2021. PMID: 34521817 Free PMC article.
References
-
- Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim. Biophys. Acta. 2009;1795:1–15. - PubMed
Grants and funding
- P30GM106397/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- R01 CA206444/CA/NCI NIH HHS/United States
- P30 GM106397/GM/NIGMS NIH HHS/United States
- P50 CA127297/CA/NCI NIH HHS/United States
- P50CA127297/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
LinkOut - more resources
Full Text Sources
Research Materials

