Altered Serum MicroRNA Profile May Serve as an Auxiliary Tool for Discriminating Aggressive Thyroid Carcinoma from Nonaggressive Thyroid Cancer and Benign Thyroid Nodules
- PMID: 31636734
- PMCID: PMC6766139
- DOI: 10.1155/2019/3717683
Altered Serum MicroRNA Profile May Serve as an Auxiliary Tool for Discriminating Aggressive Thyroid Carcinoma from Nonaggressive Thyroid Cancer and Benign Thyroid Nodules
Abstract
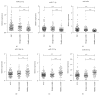
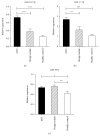
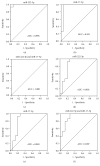
Thyroid cancers are the most common malignancy of the endocrine system; however, there is no reliable blood biomarkers for thyroid cancer diagnosis and even for aggressive and nonaggressive thyroid cancers as well as benign nodule discrimination. The present study is aimed at evaluating whether circulating microRNA (miRNA) can differentiate aggressive and nonaggressive thyroid cancer from benign thyroid nodules. In this study, we performed a multiphase, case-control study to screen serum miRNA expression profile in 100 patients with papillary thyroid cancer (PTC), 15 patients with aggressive medullary thyroid carcinoma (MTC), 91 patients with benign nodules, and 89 healthy controls using TaqMan low-density array followed by extensive reverse transcription quantitative real-time PCR validation. The results showed that the serum levels of miR-222-3p, miR-17-5p, and miR-451a were markedly increased, while miR-146a-5p, miR-132-3p, and miR-183-3p were significantly decreased in the PTC and benign nodule groups compared with the control group. There was no difference in the miRNA expression profile between the PTC group and the benign nodule group. Nevertheless, the serum levels of miR-222-3p and miR-17-5p were significantly increased in the MTC group than the benign nodule and control group. Moreover, receiver operating characteristic curve analyses demonstrated that the 2 miRNAs and their panel can accurately discriminate MTC from the benign nodule group and healthy controls. These findings indicated that the altered circulating miRNAs may discriminate PTC and benign thyroid nodules from controls, and serum miR-222-3p and miR-17-5p have the potential to serve as auxiliary tools for diagnosing more aggressive thyroid carcinomas, such as MTC.
Copyright © 2019 Aisen Zhang et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures

Similar articles
-
Circulating microRNA124-3p, microRNA9-3p and microRNA196b-5p may be potential signatures for differential diagnosis of thyroid nodules.Oncotarget. 2016 Dec 20;7(51):84165-84177. doi: 10.18632/oncotarget.12389. Oncotarget. 2016. PMID: 27705935 Free PMC article.
-
Circulating miR-25-3p and miR-451a May Be Potential Biomarkers for the Diagnosis of Papillary Thyroid Carcinoma.PLoS One. 2015 Jul 13;10(7):e0132403. doi: 10.1371/journal.pone.0132403. eCollection 2015. PLoS One. 2015. PMID: 26168287 Free PMC article.
-
Circulating microRNA profiles as potential biomarkers for diagnosis of papillary thyroid carcinoma.J Clin Endocrinol Metab. 2012 Jun;97(6):2084-92. doi: 10.1210/jc.2011-3059. Epub 2012 Apr 3. J Clin Endocrinol Metab. 2012. PMID: 22472564
-
Circulating microRNA as potential diagnostic and prognostic biomarkers of well-differentiated thyroid cancer: A review article.Cancer Biomark. 2023;36(3):193-205. doi: 10.3233/CBM-210504. Cancer Biomark. 2023. PMID: 36776042 Review.
-
miR-451a is underexpressed and targets AKT/mTOR pathway in papillary thyroid carcinoma.Oncotarget. 2016 Mar 15;7(11):12731-47. doi: 10.18632/oncotarget.7262. Oncotarget. 2016. PMID: 26871295 Free PMC article. Review.
Cited by
-
BRAFV600E Induction in Thyrocytes Triggers Important Changes in the miRNAs Content and the Populations of Extracellular Vesicles Released in Thyroid Tumor Microenvironment.Biomedicines. 2022 Mar 23;10(4):755. doi: 10.3390/biomedicines10040755. Biomedicines. 2022. PMID: 35453506 Free PMC article.
-
Identification of Cerebrospinal Fluid MicroRNAs Associated With Leptomeningeal Metastasis From Lung Adenocarcinoma.Front Oncol. 2020 Apr 3;10:387. doi: 10.3389/fonc.2020.00387. eCollection 2020. Front Oncol. 2020. PMID: 32328453 Free PMC article.
-
Translational Utility of Liquid Biopsies in Thyroid Cancer Management.Cancers (Basel). 2021 Jul 9;13(14):3443. doi: 10.3390/cancers13143443. Cancers (Basel). 2021. PMID: 34298656 Free PMC article. Review.
-
Identification of Exosomal microRNAs and Their Targets in Papillary Thyroid Cancer Cells.Biomedicines. 2022 Apr 21;10(5):961. doi: 10.3390/biomedicines10050961. Biomedicines. 2022. PMID: 35625697 Free PMC article.
-
The Blood Biomarkers of Thyroid Cancer.Cancer Manag Res. 2020 Jul 6;12:5431-5438. doi: 10.2147/CMAR.S261170. eCollection 2020. Cancer Manag Res. 2020. PMID: 32753960 Free PMC article. Review.
References
-
- Gharib H., Papini E., Paschke R., et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European thyroid association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocrine Practice. 2010;16(Supplement 1):1–43. doi: 10.4158/10024.GL. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

