Targeting cardiac fibrosis with engineered T cells
- PMID: 31511695
- PMCID: PMC6752964
- DOI: 10.1038/s41586-019-1546-z
Targeting cardiac fibrosis with engineered T cells
Erratum in
-
Author Correction: Targeting cardiac fibrosis with engineered T cells.Nature. 2019 Dec;576(7785):E2. doi: 10.1038/s41586-019-1761-7. Nature. 2019. PMID: 31723271
Abstract
Fibrosis is observed in nearly every form of myocardial disease1. Upon injury, cardiac fibroblasts in the heart begin to remodel the myocardium by depositing excess extracellular matrix, resulting in increased stiffness and reduced compliance of the tissue. Excessive cardiac fibrosis is an important factor in the progression of various forms of cardiac disease and heart failure2. However, clinical interventions and therapies that target fibrosis remain limited3. Here we demonstrate the efficacy of redirected T cell immunotherapy to specifically target pathological cardiac fibrosis in mice. We find that cardiac fibroblasts that express a xenogeneic antigen can be effectively targeted and ablated by adoptive transfer of antigen-specific CD8+ T cells. Through expression analysis of the gene signatures of cardiac fibroblasts obtained from healthy and diseased human hearts, we identify an endogenous target of cardiac fibroblasts-fibroblast activation protein. Adoptive transfer of T cells that express a chimeric antigen receptor against fibroblast activation protein results in a significant reduction in cardiac fibrosis and restoration of function after injury in mice. These results provide proof-of-principle for the development of immunotherapeutic drugs for the treatment of cardiac disease.
Figures
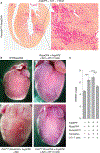
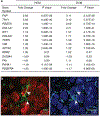


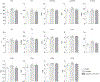
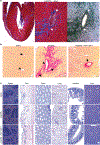






Comment in
-
CAR T cells combat cardiac fibrosis.Nat Rev Immunol. 2019 Nov;19(11):659. doi: 10.1038/s41577-019-0226-4. Nat Rev Immunol. 2019. PMID: 31551571 No abstract available.
-
CAR T cells combat cardiac fibrosis.Nat Rev Cardiol. 2019 Dec;16(12):699. doi: 10.1038/s41569-019-0287-x. Nat Rev Cardiol. 2019. PMID: 31554926 No abstract available.
-
CAR-T cells combat cardiac fibrosis.Nat Rev Drug Discov. 2019 Oct;18(11):823. doi: 10.1038/d41573-019-00162-0. Nat Rev Drug Discov. 2019. PMID: 31673127 No abstract available.
Similar articles
-
Preclinical scenario of targeting myocardial fibrosis with chimeric antigen receptor (CAR) immunotherapy.Biomed Pharmacother. 2023 Feb;158:114061. doi: 10.1016/j.biopha.2022.114061. Epub 2022 Dec 8. Biomed Pharmacother. 2023. PMID: 36495661 Review.
-
Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities.Mol Aspects Med. 2019 Feb;65:70-99. doi: 10.1016/j.mam.2018.07.001. Epub 2018 Aug 2. Mol Aspects Med. 2019. PMID: 30056242 Review.
-
CD8+-T Cells With Specificity for a Model Antigen in Cardiomyocytes Can Become Activated After Transverse Aortic Constriction but Do Not Accelerate Progression to Heart Failure.Front Immunol. 2018 Nov 15;9:2665. doi: 10.3389/fimmu.2018.02665. eCollection 2018. Front Immunol. 2018. PMID: 30498501 Free PMC article.
-
Featured Article: TGF-β1 dominates extracellular matrix rigidity for inducing differentiation of human cardiac fibroblasts to myofibroblasts.Exp Biol Med (Maywood). 2018 Apr;243(7):601-612. doi: 10.1177/1535370218761628. Epub 2018 Mar 4. Exp Biol Med (Maywood). 2018. PMID: 29504479 Free PMC article.
-
Antifibrotic response of cardiac fibroblasts in hypertensive hearts through enhanced TIMP-1 expression by basic fibroblast growth factor.Cardiovasc Pathol. 2014 Mar-Apr;23(2):92-100. doi: 10.1016/j.carpath.2013.11.001. Epub 2013 Nov 14. Cardiovasc Pathol. 2014. PMID: 24322055
Cited by
-
Renal Fibrosis in Lupus Nephritis.Int J Mol Sci. 2022 Nov 18;23(22):14317. doi: 10.3390/ijms232214317. Int J Mol Sci. 2022. PMID: 36430794 Free PMC article. Review.
-
Ligand-Induced Degradation of a CAR Permits Reversible Remote Control of CAR T Cell Activity In Vitro and In Vivo.Mol Ther. 2020 Jul 8;28(7):1600-1613. doi: 10.1016/j.ymthe.2020.06.004. Epub 2020 Jun 11. Mol Ther. 2020. PMID: 32559430 Free PMC article.
-
Towards precision medicine in heart failure.Nat Rev Cardiol. 2021 Nov;18(11):745-762. doi: 10.1038/s41569-021-00566-9. Epub 2021 Jun 9. Nat Rev Cardiol. 2021. PMID: 34108678 Review.
-
The Future of CAR T Therapeutics to Treat Autoimmune Disorders.Mol Diagn Ther. 2024 Sep;28(5):593-600. doi: 10.1007/s40291-024-00730-0. Epub 2024 Jul 30. Mol Diagn Ther. 2024. PMID: 39078456 Free PMC article. Review.
-
Emerging mRNA therapies for cardiac fibrosis.Am J Physiol Cell Physiol. 2024 Jan 1;326(1):C107-C111. doi: 10.1152/ajpcell.00504.2023. Epub 2023 Dec 4. Am J Physiol Cell Physiol. 2024. PMID: 38047297 Free PMC article.
References
Methods References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

