E-cadherin is required for metastasis in multiple models of breast cancer
- PMID: 31485072
- PMCID: PMC7365572
- DOI: 10.1038/s41586-019-1526-3
E-cadherin is required for metastasis in multiple models of breast cancer
Abstract
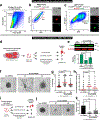
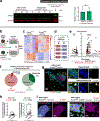
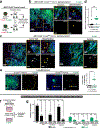
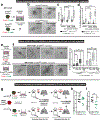
Metastasis is the major driver of death in patients with cancer. Invasion of surrounding tissues and metastasis have been proposed to initiate following loss of the intercellular adhesion protein, E-cadherin1,2, on the basis of inverse correlations between in vitro migration and E-cadherin levels3. However, this hypothesis is inconsistent with the observation that most breast cancers are invasive ductal carcinomas and express E-cadherin in primary tumours and metastases4. To resolve this discrepancy, we tested the genetic requirement for E-cadherin in metastasis using mouse and human models of both luminal and basal invasive ductal carcinomas. Here we show that E-cadherin promotes metastasis in diverse models of invasive ductal carcinomas. While loss of E-cadherin increased invasion, it also reduced cancer cell proliferation and survival, circulating tumour cell number, seeding of cancer cells in distant organs and metastasis outgrowth. Transcriptionally, loss of E-cadherin was associated with upregulation of genes involved in transforming growth factor-β (TGFβ), reactive oxygen species and apoptosis signalling pathways. At the cellular level, disseminating E-cadherin-negative cells exhibited nuclear enrichment of SMAD2/3, oxidative stress and increased apoptosis. Colony formation of E-cadherin-negative cells was rescued by inhibition of TGFβ-receptor signalling, reactive oxygen accumulation or apoptosis. Our results reveal that E-cadherin acts as a survival factor in invasive ductal carcinomas during the detachment, systemic dissemination and seeding phases of metastasis by limiting reactive oxygen-mediated apoptosis. Identifying molecular strategies to inhibit E-cadherin-mediated survival in metastatic breast cancer cells may have potential as a therapeutic approach for breast cancer.
Conflict of interest statement
The authors declare competing financial interests: details are available in the online version of the paper.
Figures
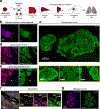

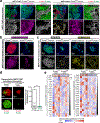

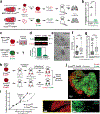
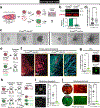



Comment in
-
Survival skills ensure that cancer spreads.Nature. 2019 Sep;573(7774):353-354. doi: 10.1038/d41586-019-02570-z. Nature. 2019. PMID: 31530905 No abstract available.
-
Cancer: Context Is Key for E-cadherin in Invasion and Metastasis.Curr Biol. 2019 Nov 4;29(21):R1140-R1142. doi: 10.1016/j.cub.2019.09.054. Curr Biol. 2019. PMID: 31689402
-
Role Reversal: A Pro-metastatic Function of E-Cadherin.Dev Cell. 2019 Nov 18;51(4):417-419. doi: 10.1016/j.devcel.2019.10.028. Dev Cell. 2019. PMID: 31743661
Similar articles
-
Expression of E-cadherin in human ductal breast cancer carcinoma in situ, invasive carcinomas, their lymph node metastases, their distant metastases, carcinomas with recurrence and in recurrence.Anticancer Res. 2007 Jul-Aug;27(4A):1969-74. Anticancer Res. 2007. PMID: 17649807
-
Expression of sialyl lewis X, sialyl Lewis A, E-cadherin and cathepsin-D in human breast cancer: immunohistochemical analysis in mammary carcinoma in situ, invasive carcinomas and their lymph node metastasis.Anticancer Res. 2005 May-Jun;25(3A):1615-22. Anticancer Res. 2005. PMID: 16033070
-
Re-Evaluating E-Cadherin and β-Catenin: A Pan-Cancer Proteomic Approach with an Emphasis on Breast Cancer.Am J Pathol. 2018 Aug;188(8):1910-1920. doi: 10.1016/j.ajpath.2018.05.003. Epub 2018 Jun 4. Am J Pathol. 2018. PMID: 29879416 Free PMC article.
-
Transforming growth factor-beta signaling: emerging stem cell target in metastatic breast cancer?Breast Cancer Res Treat. 2009 Jun;115(3):453-95. doi: 10.1007/s10549-008-0184-1. Epub 2008 Oct 9. Breast Cancer Res Treat. 2009. PMID: 18841463 Free PMC article. Review.
-
The role of cadherin-mediated adhesion in breast cancer.J Mammary Gland Biol Neoplasia. 1996 Apr;1(2):219-29. doi: 10.1007/BF02013645. J Mammary Gland Biol Neoplasia. 1996. PMID: 10887495 Review.
Cited by
-
Organotypic culture assays for murine and human primary and metastatic-site tumors.Nat Protoc. 2020 Aug;15(8):2413-2442. doi: 10.1038/s41596-020-0335-3. Epub 2020 Jul 20. Nat Protoc. 2020. PMID: 32690957 Free PMC article.
-
Dissecting FGF Signalling to Target Cellular Crosstalk in Pancreatic Cancer.Cells. 2021 Apr 8;10(4):847. doi: 10.3390/cells10040847. Cells. 2021. PMID: 33918004 Free PMC article. Review.
-
Colonization of distant organs by tumor cells generating circulating homotypic clusters adaptive to fluid shear stress.Sci Rep. 2021 Mar 17;11(1):6150. doi: 10.1038/s41598-021-85743-z. Sci Rep. 2021. PMID: 33731803 Free PMC article.
-
Cadherin Expression Profiles Define Glioblastoma Differentiation and Patient Prognosis.Cancers (Basel). 2024 Jun 22;16(13):2298. doi: 10.3390/cancers16132298. Cancers (Basel). 2024. PMID: 39001361 Free PMC article.
-
Impact of RBMS 3 Progression on Expression of EMT Markers.Cells. 2024 Sep 14;13(18):1548. doi: 10.3390/cells13181548. Cells. 2024. PMID: 39329736 Free PMC article.
References
REFERENCES (METHODS):
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

