Non-canonical mTORC2 Signaling Regulates Brown Adipocyte Lipid Catabolism through SIRT6-FoxO1
- PMID: 31442424
- PMCID: PMC7388077
- DOI: 10.1016/j.molcel.2019.07.023
Non-canonical mTORC2 Signaling Regulates Brown Adipocyte Lipid Catabolism through SIRT6-FoxO1
Abstract
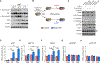
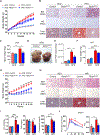
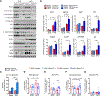
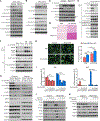
mTORC2 controls glucose and lipid metabolism, but the mechanisms are unclear. Here, we show that conditionally deleting the essential mTORC2 subunit Rictor in murine brown adipocytes inhibits de novo lipid synthesis, promotes lipid catabolism and thermogenesis, and protects against diet-induced obesity and hepatic steatosis. AKT kinases are the canonical mTORC2 substrates; however, deleting Rictor in brown adipocytes appears to drive lipid catabolism by promoting FoxO1 deacetylation independently of AKT, and in a pathway distinct from its positive role in anabolic lipid synthesis. This facilitates FoxO1 nuclear retention, enhances lipid uptake and lipolysis, and potentiates UCP1 expression. We provide evidence that SIRT6 is the FoxO1 deacetylase suppressed by mTORC2 and show an endogenous interaction between SIRT6 and mTORC2 in both mouse and human cells. Our findings suggest a new paradigm of mTORC2 function filling an important gap in our understanding of this more mysterious mTOR complex.
Keywords: ATGL; FoxO1; Rictor; Sirt6; UCP1; acetylation; adipocyte; brown adipose tissue; brown fat; lipid; mTOR; mTORC2; metabolism; signaling.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interests
The authors declare no conflicts of interest.
Figures


Similar articles
-
Rictor, an mTORC2 Protein, Regulates Murine Lymphatic Valve Formation Through the AKT-FOXO1 Signaling.Arterioscler Thromb Vasc Biol. 2024 Sep;44(9):2004-2023. doi: 10.1161/ATVBAHA.124.321164. Epub 2024 Aug 1. Arterioscler Thromb Vasc Biol. 2024. PMID: 39087350 Free PMC article.
-
Proteome and Phosphoproteome Analysis of Brown Adipocytes Reveals That RICTOR Loss Dampens Global Insulin/AKT Signaling.Mol Cell Proteomics. 2020 Jul;19(7):1104-1119. doi: 10.1074/mcp.RA120.001946. Epub 2020 Mar 31. Mol Cell Proteomics. 2020. PMID: 32234964 Free PMC article.
-
Enhancing Acsl4 in absence of mTORC2/Rictor drove β-cell dedifferentiation via inhibiting FoxO1 and promoting ROS production.Biochim Biophys Acta Mol Basis Dis. 2021 Dec 1;1867(12):166261. doi: 10.1016/j.bbadis.2021.166261. Epub 2021 Aug 27. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 34455055
-
Unmasking the impact of Rictor in cancer: novel insights of mTORC2 complex.Carcinogenesis. 2018 Jul 30;39(8):971-980. doi: 10.1093/carcin/bgy086. Carcinogenesis. 2018. PMID: 29955840 Review.
-
Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship.Adv Biol Regul. 2019 May;72:51-62. doi: 10.1016/j.jbior.2019.03.003. Epub 2019 Apr 11. Adv Biol Regul. 2019. PMID: 31010692 Review.
Cited by
-
SIRT6 Widely Regulates Aging, Immunity, and Cancer.Front Oncol. 2022 Apr 6;12:861334. doi: 10.3389/fonc.2022.861334. eCollection 2022. Front Oncol. 2022. PMID: 35463332 Free PMC article. Review.
-
The widely used Ucp1-CreEvdr transgene elicits complex developmental and metabolic phenotypes.bioRxiv [Preprint]. 2023 Oct 20:2023.10.20.563165. doi: 10.1101/2023.10.20.563165. bioRxiv. 2023. Update in: Nat Commun. 2025 Jan 17;16(1):770. doi: 10.1038/s41467-024-54763-4. PMID: 37904917 Free PMC article. Updated. Preprint.
-
mTORC2: The other mTOR in autophagy regulation.Aging Cell. 2021 Aug;20(8):e13431. doi: 10.1111/acel.13431. Epub 2021 Jul 12. Aging Cell. 2021. PMID: 34250734 Free PMC article. Review.
-
The central moTOR of metabolism.Dev Cell. 2022 Mar 28;57(6):691-706. doi: 10.1016/j.devcel.2022.02.024. Epub 2022 Mar 21. Dev Cell. 2022. PMID: 35316619 Free PMC article. Review.
-
Sestrin2 in diabetes and diabetic complications.Front Endocrinol (Lausanne). 2023 Oct 18;14:1274686. doi: 10.3389/fendo.2023.1274686. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37920252 Free PMC article. Review.
References
-
- BARQUISSAU V, BEUZELIN D, PISANI DF, BERANGER GE, MAIRAL A, MONTAGNER A, ROUSSEL B, TAVERNIER G, MARQUES MA, MORO C, GUILLOU H, AMRI EZ & LANGIN D. 2016. White-to-brite conversion in human adipocytes promotes metabolic reprogramming towards fatty acid anabolic and catabolic pathways. Mol Metab, 5, 352–365. - PMC - PubMed
-
- CANNON B. & NEDERGAARD J. 2004. Brown adipose tissue: function and physiological significance. Physiol Rev, 84, 277–359. - PubMed
-
- CANNON B. & NEDERGAARD J. 2011. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol, 214, 242–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

