Mechanism for Regulation of Melanoma Cell Death via Activation of Thermo-TRPV4 and TRPV2
- PMID: 30881453
- PMCID: PMC6383420
- DOI: 10.1155/2019/7362875
Mechanism for Regulation of Melanoma Cell Death via Activation of Thermo-TRPV4 and TRPV2
Abstract
Background: Thermo-TRPs (temperature-sensitive transient receptor potential channels) belong to the TRP (transient receptor potential) channel superfamily. Emerging evidence implied that thermo-TRPs have been involved in regulation of cell fate in certain tumors. However, their distribution profiles and roles in melanoma remain incompletely understood.
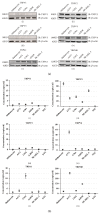
Methods: Western blot and digital PCR approaches were performed to identify the distribution profiles of six thermo-TRPs. MTT assessment was employed to detect cell viability. Flow cytometry was applied to test cell cycle and apoptosis. Calcium imaging was used to determine the function of channels. Five cell lines, including one normal human primary epidermal melanocytes and two human malignant melanoma (A375, G361) and two human metastatic melanoma (A2058, SK-MEL-3) cell lines, were chosen for this research.
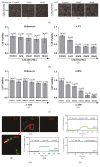
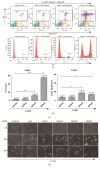
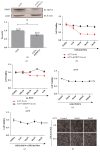
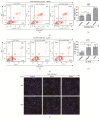
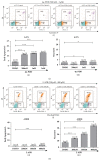
Results: In the present study, six thermo-TRPs including TRPV1/2/3/4, TRPA1, and TRPM8 were examined in human primary melanocytes and melanoma cells. We found that TRPV2/4, TRPA1, and TRPM8 exhibited ectopic distribution both in melanocytes and melanoma cells. Moreover, activation of TRPV2 and TRPV4 could lead to the decline of cell viability for melanoma A2058 and A375 cells. Subsequently, activation of TRPV2 by 2-APB (IC50 = 150 μM) induced cell necrosis in A2058 cells, while activation of TRPV4 by GSK1016790A (IC50 = 10 nM) enhanced apoptosis of A375 cells. Furthermore, TRPV4 mediated cell apoptosis of melanoma via phosphorylation of AKT and was involved in calcium regulation.
Conclusion: Overall, our studies revealed that TRPV4 and TRPV2 mediated melanoma cell death via channel activation and characterized the mechanism of functional TRPV4 ion channel in regulating AKT pathway driven antitumor process. Thus, they may serve as potential biomarkers for the prognosis and are targeted for the therapeutic use in human melanoma.
Figures

Similar articles
-
Pharmacological activation of TRPV4 produces immediate cell damage and induction of apoptosis in human melanoma cells and HaCaT keratinocytes.PLoS One. 2018 Jan 2;13(1):e0190307. doi: 10.1371/journal.pone.0190307. eCollection 2018. PLoS One. 2018. PMID: 29293584 Free PMC article.
-
Functional expression of transient receptor potential channels in human endometrial stromal cells during the luteal phase of the menstrual cycle.Hum Reprod. 2015 Jun;30(6):1421-36. doi: 10.1093/humrep/dev068. Epub 2015 Mar 27. Hum Reprod. 2015. PMID: 25820697
-
TNF-α differently regulates TRPV2 and TRPV4 channels in human dental pulp cells.Int Endod J. 2019 Nov;52(11):1617-1628. doi: 10.1111/iej.13174. Epub 2019 Jul 8. Int Endod J. 2019. PMID: 31206742
-
Temperature-sensitive transient receptor potential channels in corneal tissue layers and cells.Ophthalmic Res. 2014;52(3):151-9. doi: 10.1159/000365334. Epub 2014 Oct 8. Ophthalmic Res. 2014. PMID: 25301091 Review.
-
Thermo-Sensitive TRP Channels: Novel Targets for Treating Chemotherapy-Induced Peripheral Pain.Front Physiol. 2017 Dec 13;8:1040. doi: 10.3389/fphys.2017.01040. eCollection 2017. Front Physiol. 2017. PMID: 29326595 Free PMC article. Review.
Cited by
-
Role of Transient Receptor Potential Vanilloid 4 Channel in Skin Physiology and Pathology.Sultan Qaboos Univ Med J. 2020 May;20(2):e138-e146. doi: 10.18295/squmj.2020.20.02.003. Epub 2020 Jun 28. Sultan Qaboos Univ Med J. 2020. PMID: 32655905 Free PMC article. Review.
-
Heteromeric TRP Channels in Lung Inflammation.Cells. 2021 Jul 1;10(7):1654. doi: 10.3390/cells10071654. Cells. 2021. PMID: 34359824 Free PMC article. Review.
-
Identification of TUBB4A as a Prognostic Biomarker of Melanoma by Transcriptomic Data and In Vitro Experiments.Technol Cancer Res Treat. 2023 Jan-Dec;22:15330338231184842. doi: 10.1177/15330338231184842. Technol Cancer Res Treat. 2023. PMID: 37439014 Free PMC article.
-
The Role of Cannabidiol in Liver Disease: A Systemic Review.Int J Mol Sci. 2024 Feb 17;25(4):2370. doi: 10.3390/ijms25042370. Int J Mol Sci. 2024. PMID: 38397045 Free PMC article. Review.
-
TRP Channels in Cancer: Signaling Mechanisms and Translational Approaches.Biomolecules. 2023 Oct 22;13(10):1557. doi: 10.3390/biom13101557. Biomolecules. 2023. PMID: 37892239 Free PMC article. Review.
References
-
- Zhou Z. H., Song J. W., Li W., et al. The acid-sensing ion channel, ASIC2, promotes invasion and metastasis of colorectal cancer under acidosis by activating the calcineurin/NFAT1 axis. Journal of Experimental & Clinical Cancer Research. 2017;36(130):1–12. doi: 10.1186/s13046-016-0484-y. - DOI - PMC - PubMed
-
- Fraser S. P., Grimes J. A., Djamgoz M. B. A., et al. Effects of voltage-gated ion channel modulators on rat prostatic cancer cell proliferation: Comparison of strongly and weakly metastatic cell lines. The Prostate. 2000;44(1):61–76. doi: 10.1002/1097-0045(20000615)44:1<61::AID-PROS9>3.0.CO;2-3. - DOI - PubMed
LinkOut - more resources
Full Text Sources

