Human pluripotent stem cell-derived models and drug screening in CNS precision medicine
- PMID: 30875083
- PMCID: PMC8193821
- DOI: 10.1111/nyas.14012
Human pluripotent stem cell-derived models and drug screening in CNS precision medicine
Abstract
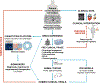
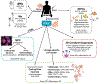
Development of effective therapeutics for neurological disorders has historically been challenging partly because of lack of accurate model systems in which to investigate disease etiology and test new therapeutics at the preclinical stage. Human stem cells, particularly patient-derived induced pluripotent stem cells (iPSCs) upon differentiation, have the ability to recapitulate aspects of disease pathophysiology and are increasingly recognized as robust scalable systems for drug discovery. We review advances in deriving cellular models of human central nervous system (CNS) disorders using iPSCs along with strategies for investigating disease-relevant phenotypes, translatable biomarkers, and therapeutic targets. Given their potential to identify novel therapeutic targets and leads, we focus on phenotype-based, small-molecule screens employing human stem cell-derived models. Integrated efforts to assemble patient iPSC-derived cell models with deeply annotated clinicopathological data, along with molecular and drug-response signatures, may aid in the stratification of patients, diagnostics, and clinical trial success, shifting translational science and precision medicine approaches. A number of remaining challenges, including the optimization of cost-effective, large-scale culture of iPSC-derived cell types, incorporation of aging into neuronal models, as well as robustness and automation of phenotypic assays to support quantitative drug efficacy, toxicity, and metabolism testing workflows, are covered. Continued advancement of the field is expected to help fully humanize the process of CNS drug discovery.
Keywords: drug discovery; human-induced pluripotent stem cells; neurodegenerative disorders; neuroscience; psychiatric disorders; screening.
© 2019 New York Academy of Sciences.
Conflict of interest statement
Competing Interests
SJH is a scientific advisory board member of Rodin Therapeutics, Psy Therapeutics, and Frequency Therapeutics. None of these entities were involved in manuscript preparation.
Figures


Similar articles
-
Advancing drug discovery for neuropsychiatric disorders using patient-specific stem cell models.Mol Cell Neurosci. 2016 Jun;73:104-15. doi: 10.1016/j.mcn.2016.01.011. Epub 2016 Jan 28. Mol Cell Neurosci. 2016. PMID: 26826498 Free PMC article. Review.
-
Human iPSC-Based Modeling of Central Nerve System Disorders for Drug Discovery.Int J Mol Sci. 2021 Jan 26;22(3):1203. doi: 10.3390/ijms22031203. Int J Mol Sci. 2021. PMID: 33530458 Free PMC article. Review.
-
Induced Pluripotent Stem Cell (iPSC)-Based Neurodegenerative Disease Models for Phenotype Recapitulation and Drug Screening.Molecules. 2020 Apr 24;25(8):2000. doi: 10.3390/molecules25082000. Molecules. 2020. PMID: 32344649 Free PMC article. Review.
-
Concise review: modeling central nervous system diseases using induced pluripotent stem cells.Stem Cells Transl Med. 2014 Dec;3(12):1418-28. doi: 10.5966/sctm.2014-0102. Epub 2014 Nov 3. Stem Cells Transl Med. 2014. PMID: 25368377 Free PMC article. Review.
-
Differentiation of Human Induced Pluripotent Stem Cells into Cortical Neurons to Advance Precision Medicine.Methods Mol Biol. 2022;2429:143-174. doi: 10.1007/978-1-0716-1979-7_10. Methods Mol Biol. 2022. PMID: 35507160
Cited by
-
Genome-wide CRISPR screen identifies protein pathways modulating tau protein levels in neurons.Commun Biol. 2021 Jun 14;4(1):736. doi: 10.1038/s42003-021-02272-1. Commun Biol. 2021. PMID: 34127790 Free PMC article.
-
Comparing stem cells, transdifferentiation and brain organoids as tools for psychiatric research.Transl Psychiatry. 2024 Feb 28;14(1):127. doi: 10.1038/s41398-024-02780-8. Transl Psychiatry. 2024. PMID: 38418498 Free PMC article. Review.
-
The Clinical Trials of Mesenchymal Stromal Cells Therapy.Stem Cells Int. 2021 Nov 3;2021:1634782. doi: 10.1155/2021/1634782. eCollection 2021. Stem Cells Int. 2021. PMID: 34745268 Free PMC article. Review.
-
hiPSC-Derived Neurons Provide a Robust and Physiologically Relevant In Vitro Platform to Test Botulinum Neurotoxins.Front Pharmacol. 2021 Jan 14;11:617867. doi: 10.3389/fphar.2020.617867. eCollection 2020. Front Pharmacol. 2021. PMID: 33519485 Free PMC article.
-
Integrating CRISPR Engineering and hiPSC-Derived 2D Disease Modeling Systems.J Neurosci. 2020 Feb 5;40(6):1176-1185. doi: 10.1523/JNEUROSCI.0518-19.2019. J Neurosci. 2020. PMID: 32024766 Free PMC article. Review.
References
-
- Arneric SP, Kern VD&Stephenson DT 2018. Regulatory-accepted drug development tools are needed to accelerate innovative CNS disease treatments. Biochem Pharmacol. 151: 291–306. - PubMed
-
- Kinch MS 2015. An analysis of FDA-approved drugs for neurological disorders. Drug Discov Today. 20: 1040–1043. - PubMed
-
- Association., A. s. 2016. Alzheimer’s Disease Facts and Figures. - PubMed
Publication types
MeSH terms
Grants and funding
- R01MH091115/MH/NIMH NIH HHS/United States
- R01 MH091115/MH/NIMH NIH HHS/United States
- R33MH087896/MH/NIMH NIH HHS/United States
- R01 AT009144/AT/NCCIH NIH HHS/United States
- P50 MH106933/MH/NIMH NIH HHS/United States
- R01 MH095088/MH/NIMH NIH HHS/United States
- RF1 AG042978/AG/NIA NIH HHS/United States
- R01AT009144/AT/NCCIH NIH HHS/United States
- R21 NS085487/NS/NINDS NIH HHS/United States
- P50MH106933/HG/NHGRI NIH HHS/United States
- R33 MH087896/MH/NIMH NIH HHS/United States
- R01NS108115/NS/NINDS NIH HHS/United States
- R01 NS108115/NS/NINDS NIH HHS/United States
- R21NS085487/NS/NINDS NIH HHS/United States
- R01MH095088/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

