MicroRNA-26a/Death-Associated Protein Kinase 1 Signaling Induces Synucleinopathy and Dopaminergic Neuron Degeneration in Parkinson's Disease
- PMID: 30718039
- PMCID: PMC8861874
- DOI: 10.1016/j.biopsych.2018.12.008
MicroRNA-26a/Death-Associated Protein Kinase 1 Signaling Induces Synucleinopathy and Dopaminergic Neuron Degeneration in Parkinson's Disease
Abstract
Background: Death-associated protein kinase 1 (DAPK1) is a widely distributed serine/threonine kinase that is critical for cell death in multiple neurological disorders, including Alzheimer's disease and stroke. However, little is known about the role of DAPK1 in the pathogenesis of Parkinson's disease (PD), the second most common neurodegenerative disorder.
Methods: We used Western blot and immunohistochemistry to evaluate the alteration of DAPK1. Quantitative polymerase chain reaction and fluorescence in situ hybridization were used to analyze the expression of microRNAs in PD mice and patients with PD. Rotarod, open field, and pole tests were used to evaluate the locomotor ability. Immunofluorescence, Western blot, and filter traps were used to evaluate synucleinopathy in PD mice.
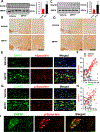
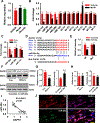
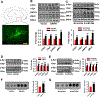
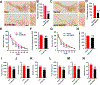
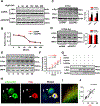
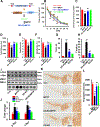
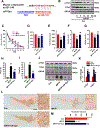
Results: We found that DAPK1 is posttranscriptionally upregulated by a reduction in microRNA-26a (miR-26a) caused by a loss of the transcription factor CCAAT enhancer-binding protein alpha. The overexpression of DAPK1 in PD mice is positively correlated with neuronal synucleinopathy. Suppressing miR-26a or upregulating DAPK1 results in synucleinopathy, dopaminergic neuron cell death, and motor disabilities in wild-type mice. In contrast, genetic deletion of DAPK1 in dopaminergic neurons by crossing DAT-Cre mice with DAPK1 floxed mice effectively rescues the abnormalities in mice with chronic MPTP treatment. We further showed that DAPK1 overexpression promotes PD-like phenotypes by direct phosphorylation of α-synuclein at the serine 129 site. Correspondingly, a cell-permeable competing peptide that blocks the phosphorylation of α-synuclein prevents motor disorders, synucleinopathy, and dopaminergic neuron loss in the MPTP mice.
Conclusions: miR-26a/DAPK1 signaling cascades are essential in the formation of the molecular and cellular pathologies in PD.
Keywords: DAPK1; MPTP; Parkinson’s disease; Peptide; miRNA; α-Synuclein.
Copyright © 2018 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest
The peptide used to block synuclein phosphorylation submitted to the Patent Office of the People’s Republic of China by Dr. Ling-Qiang Zhu, Dr. Man-Fei Deng, Dr. Dan Liu, Dr. Ao-Ji Xie and Dr.Ya-Fan Zhou (Application No. 2018100396990). All other authors report no biomedical financial interests or potential conflicts of interest.
Figures
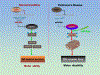
Similar articles
-
Circular RNA circTLK1 regulates dopaminergic neuron injury during Parkinson's disease by targeting miR-26a-5p/DAPK1.Neurosci Lett. 2022 Jun 21;782:136638. doi: 10.1016/j.neulet.2022.136638. Epub 2022 Apr 18. Neurosci Lett. 2022. PMID: 35447224
-
Oxytocin Alleviates MPTP-Induced Neurotoxicity in Mice by Targeting MicroRNA-26a/Death-Associated Protein Kinase 1 Pathway.J Alzheimers Dis. 2020;74(3):883-901. doi: 10.3233/JAD-191091. J Alzheimers Dis. 2020. PMID: 32083584
-
Neuroprotective effects of osmotin in Parkinson's disease-associated pathology via the AdipoR1/MAPK/AMPK/mTOR signaling pathways.J Biomed Sci. 2023 Aug 11;30(1):66. doi: 10.1186/s12929-023-00961-z. J Biomed Sci. 2023. PMID: 37568205 Free PMC article.
-
RGS Proteins as Critical Regulators of Motor Function and Their Implications in Parkinson's Disease.Mol Pharmacol. 2020 Dec;98(6):730-738. doi: 10.1124/mol.119.118836. Epub 2020 Feb 3. Mol Pharmacol. 2020. PMID: 32015009 Free PMC article. Review.
-
Cellular models for Parkinson's disease.J Neurochem. 2016 Oct;139 Suppl 1:121-130. doi: 10.1111/jnc.13618. Epub 2016 Apr 18. J Neurochem. 2016. PMID: 27091001 Review.
Cited by
-
Exosomes derived from microglia overexpressing miR-124-3p alleviate neuronal endoplasmic reticulum stress damage after repetitive mild traumatic brain injury.Neural Regen Res. 2024 Sep 1;19(9):2010-2018. doi: 10.4103/1673-5374.391189. Epub 2023 Dec 21. Neural Regen Res. 2024. PMID: 38227530 Free PMC article.
-
MicroRNA-218-5p-Ddx41 axis restrains microglia-mediated neuroinflammation through downregulating type I interferon response in a mouse model of Parkinson's disease.J Transl Med. 2024 Jan 16;22(1):63. doi: 10.1186/s12967-024-04881-w. J Transl Med. 2024. PMID: 38229084 Free PMC article.
-
MicroRNAs in Parkinson's disease: a systematic review and diagnostic accuracy meta-analysis.Sci Rep. 2023 Sep 28;13(1):16272. doi: 10.1038/s41598-023-43096-9. Sci Rep. 2023. PMID: 37770507 Free PMC article.
-
miR-425 deficiency promotes necroptosis and dopaminergic neurodegeneration in Parkinson's disease.Cell Death Dis. 2019 Aug 5;10(8):589. doi: 10.1038/s41419-019-1809-5. Cell Death Dis. 2019. PMID: 31383850 Free PMC article.
-
Somatic and germline mutations in the tumor suppressor gene PARK2 impair PINK1/Parkin-mediated mitophagy in lung cancer cells.Acta Pharmacol Sin. 2020 Jan;41(1):93-100. doi: 10.1038/s41401-019-0260-6. Epub 2019 Jul 8. Acta Pharmacol Sin. 2020. PMID: 31285534 Free PMC article.
References
-
- Michel PP, Hirsch EC, Hunot S (2016): Understanding Dopaminergic Cell Death Pathways in Parkinson Disease. Neuron 90:675–691. - PubMed
-
- Dexter DT, Jenner P (2013): Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med 62:132–144. - PubMed
-
- Bialik S, Kimchi A (2006): The death-associated protein kinases: structure, function, and beyond. Annu Rev Biochem 75:189–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

