Natural Product Triterpenoids and Their Semi-Synthetic Derivatives with Potential Anticancer Activity
- PMID: 30658371
- PMCID: PMC6639164
- DOI: 10.1055/a-0832-2383
Natural Product Triterpenoids and Their Semi-Synthetic Derivatives with Potential Anticancer Activity
Abstract
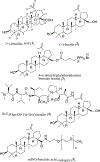
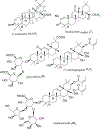
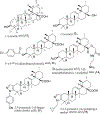
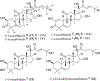
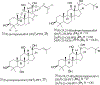
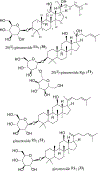
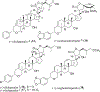
Triterpenoids are distributed widely in higher plants and are of interest because of their structural diversity and broad range of bioactivities. In particular, there is a very large literature on the propensity of a variety of triterpenoids to act as potential anticancer agents. In the present review, the anticancer potential is summarized for naturally occurring triterpenoids and their semi-synthetic derivatives, including examples of lupane-, oleanane-, ursane-, and cucurbitane-type pentacyclic triterpenoids, along with dammarane-type tetracyclic triterpenes including ginsenosides and their sapogenins and dichapetalins, which have been characterized as antitumor leads from higher plants. Preliminary structure-activity relationships and reported mechanisms of the antineoplastic-related activity are included. Prior studies for triterpenoids of plant origin are supportive of additional work being conducted on the more detailed biological and mechanistic evaluation for the progression of this type of natural products as possible cancer chemotherapeutic agents.
Georg Thieme Verlag KG Stuttgart · New York.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures
Similar articles
-
Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: Recent advances in cancer treatment.Eur J Med Chem. 2017 Dec 15;142:95-130. doi: 10.1016/j.ejmech.2017.07.013. Epub 2017 Jul 14. Eur J Med Chem. 2017. PMID: 28754470 Review.
-
Known Triterpenes and their Derivatives as Scaffolds for the Development of New Therapeutic Agents for Cancer.Curr Med Chem. 2018;25(10):1259-1269. doi: 10.2174/0929867324666170818111933. Curr Med Chem. 2018. PMID: 28820068 Review.
-
Plant-derived triterpenoids as potential antineoplastic agents.Mini Rev Med Chem. 2003 Sep;3(6):540-56. doi: 10.2174/1389557033487854. Mini Rev Med Chem. 2003. PMID: 12871157 Review.
-
Recent Advances in Antiviral Activities of Triterpenoids.Pharmaceuticals (Basel). 2022 Sep 21;15(10):1169. doi: 10.3390/ph15101169. Pharmaceuticals (Basel). 2022. PMID: 36297280 Free PMC article. Review.
-
[Biological activity and pharmacological prospects of lupane terpenoids: I. Natural lupane derivatives].Bioorg Khim. 2006 Jan-Feb;32(1):42-55. doi: 10.1134/s1068162006010031. Bioorg Khim. 2006. PMID: 16523720 Review. Russian.
Cited by
-
Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health.Molecules. 2022 Nov 13;27(22):7823. doi: 10.3390/molecules27227823. Molecules. 2022. PMID: 36431924 Free PMC article. Review.
-
Unraveling the Impact of Six Pentacyclic Triterpenes Regulating Metabolic Pathways on Lung Carcinoma Cells.Pharmaceuticals (Basel). 2024 May 28;17(6):694. doi: 10.3390/ph17060694. Pharmaceuticals (Basel). 2024. PMID: 38931361 Free PMC article.
-
Cucurbitacins as Potent Chemo-Preventive Agents: Mechanistic Insight and Recent Trends.Biomolecules. 2022 Dec 27;13(1):57. doi: 10.3390/biom13010057. Biomolecules. 2022. PMID: 36671442 Free PMC article. Review.
-
Novel Triterpenic Acid-Benzotriazole Esters Act as Pro-Apoptotic Antimelanoma Agents.Int J Mol Sci. 2022 Sep 1;23(17):9992. doi: 10.3390/ijms23179992. Int J Mol Sci. 2022. PMID: 36077389 Free PMC article.
-
Ursolic and Oleanolic Acids Induce Mitophagy in A549 Human Lung Cancer Cells.Molecules. 2019 Sep 23;24(19):3444. doi: 10.3390/molecules24193444. Molecules. 2019. PMID: 31547522 Free PMC article.
References
-
- Cragg GM, Kingston DGI, Newman DJ. (Eds.). Anticancer Agents from Natural Products, 2nd Edition; CRC Press/Taylor & Francis; Boca Raton, FL, USA, 2012.
-
- Butler MS, Robertson AAB, Cooper MA. Natural product and natural product derived drugs in clinical trials. Nat Prod Res 2014; 31: 1612–1661. - PubMed
-
- Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 2016; 79: 629–661. - PubMed
-
- Xu R, Fazio GC, Matsuda SPT. On the origins of triterpenoid skeletal diversity. Phytochemistry 2004; 65: 261–291. - PubMed
-
- Petronelli A, Pannitteri G, Testa U. Triterpenoids as new promising anticancer drugs. Anti-Cancer Drugs 2009; 20: 880–892. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

