Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction
- PMID: 30643288
- PMCID: PMC6367058
- DOI: 10.1038/s41591-018-0297-y
Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction
Abstract
Vascular contributions to cognitive impairment are increasingly recognized1-5 as shown by neuropathological6,7, neuroimaging4,8-11, and cerebrospinal fluid biomarker4,12 studies. Moreover, small vessel disease of the brain has been estimated to contribute to approximately 50% of all dementias worldwide, including those caused by Alzheimer's disease (AD)3,4,13. Vascular changes in AD have been typically attributed to the vasoactive and/or vasculotoxic effects of amyloid-β (Aβ)3,11,14, and more recently tau15. Animal studies suggest that Aβ and tau lead to blood vessel abnormalities and blood-brain barrier (BBB) breakdown14-16. Although neurovascular dysfunction3,11 and BBB breakdown develop early in AD1,4,5,8-10,12,13, how they relate to changes in the AD classical biomarkers Aβ and tau, which also develop before dementia17, remains unknown. To address this question, we studied brain capillary damage using a novel cerebrospinal fluid biomarker of BBB-associated capillary mural cell pericyte, soluble platelet-derived growth factor receptor-β8,18, and regional BBB permeability using dynamic contrast-enhanced magnetic resonance imaging8-10. Our data show that individuals with early cognitive dysfunction develop brain capillary damage and BBB breakdown in the hippocampus irrespective of Alzheimer's Aβ and/or tau biomarker changes, suggesting that BBB breakdown is an early biomarker of human cognitive dysfunction independent of Aβ and tau.
Conflict of interest statement
COMPETING INTERESTS STATEMENT:
The authors declare no competing financial interests.
Figures
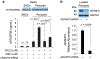
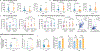
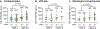



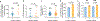


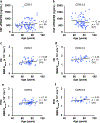
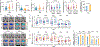

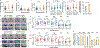

Comment in
-
Biomarkers for Alzheimer's disease beyond amyloid and tau.Nat Med. 2019 Feb;25(2):201-203. doi: 10.1038/s41591-019-0348-z. Nat Med. 2019. PMID: 30728536 No abstract available.
Similar articles
-
Assessing blood-brain barrier dysfunction and its association with Alzheimer's pathology, cognitive impairment and neuroinflammation.Alzheimers Res Ther. 2024 Jul 31;16(1):172. doi: 10.1186/s13195-024-01529-1. Alzheimers Res Ther. 2024. PMID: 39085945 Free PMC article.
-
APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline.Nature. 2020 May;581(7806):71-76. doi: 10.1038/s41586-020-2247-3. Epub 2020 Apr 29. Nature. 2020. PMID: 32376954 Free PMC article.
-
A novel sensitive assay for detection of a biomarker of pericyte injury in cerebrospinal fluid.Alzheimers Dement. 2020 Jun;16(6):821-830. doi: 10.1002/alz.12061. Epub 2020 Apr 16. Alzheimers Dement. 2020. PMID: 32301266 Free PMC article.
-
Biochemical markers in vascular cognitive impairment associated with subcortical small vessel disease - A consensus report.BMC Neurol. 2017 May 23;17(1):102. doi: 10.1186/s12883-017-0877-3. BMC Neurol. 2017. PMID: 28535786 Free PMC article. Review.
-
Simultaneous analysis of cerebrospinal fluid biomarkers using microsphere-based xMAP multiplex technology for early detection of Alzheimer's disease.Methods. 2012 Apr;56(4):484-93. doi: 10.1016/j.ymeth.2012.03.023. Epub 2012 Apr 6. Methods. 2012. PMID: 22503777 Review.
Cited by
-
Cerebrovascular Senescence Is Associated With Tau Pathology in Alzheimer's Disease.Front Neurol. 2020 Sep 16;11:575953. doi: 10.3389/fneur.2020.575953. eCollection 2020. Front Neurol. 2020. PMID: 33041998 Free PMC article.
-
Cell chirality in cardiovascular development and disease.APL Bioeng. 2020 Aug 25;4(3):031503. doi: 10.1063/5.0014424. eCollection 2020 Sep. APL Bioeng. 2020. PMID: 32903894 Free PMC article. Review.
-
Association of Cerebral Hypoperfusion and Poor Collaterals with Cognitive Impairment in Patients with Severe Vertebrobasilar Artery Stenosis.J Alzheimers Dis Rep. 2024 Jun 21;8(1):999-1007. doi: 10.3233/ADR-240007. eCollection 2024. J Alzheimers Dis Rep. 2024. PMID: 39114550 Free PMC article.
-
The brains of aged mice are characterized by altered tissue diffusion properties and cerebral microbleeds.J Transl Med. 2020 Jul 8;18(1):277. doi: 10.1186/s12967-020-02441-6. J Transl Med. 2020. PMID: 32641073 Free PMC article.
-
Citrus Polyphenols in Brain Health and Disease: Current Perspectives.Front Neurosci. 2021 Feb 19;15:640648. doi: 10.3389/fnins.2021.640648. eCollection 2021. Front Neurosci. 2021. PMID: 33679318 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG023084/AG/NIA NIH HHS/United States
- RF1 AG039452/AG/NIA NIH HHS/United States
- R01 NS090904/NS/NINDS NIH HHS/United States
- R01 NS100459/NS/NINDS NIH HHS/United States
- R01 AG054434/AG/NIA NIH HHS/United States
- R01 AG055770/AG/NIA NIH HHS/United States
- R21 AG055034/AG/NIA NIH HHS/United States
- R00 AG058780/AG/NIA NIH HHS/United States
- P01 AG052350/AG/NIA NIH HHS/United States
- P50 AG005142/AG/NIA NIH HHS/United States
- R01 AG039452/AG/NIA NIH HHS/United States
- K99 AG058780/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- R01 NS034467/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

