The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-Mediated Regulation of WNT10B Expression
- PMID: 30446387
- PMCID: PMC6345170
- DOI: 10.1016/j.immuni.2018.10.013
The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-Mediated Regulation of WNT10B Expression
Abstract
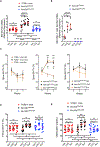
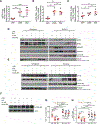
Nutritional supplementation with probiotics can prevent pathologic bone loss. Here we examined the impact of supplementation with Lactobacillus rhamnosus GG (LGG) on bone homeostasis in eugonadic young mice. Micro-computed tomography revealed that LGG increased trabecular bone volume in mice, which was due to increased bone formation. Butyrate produced in the gut following LGG ingestion, or butyrate fed directly to germ-free mice, induced the expansion of intestinal and bone marrow (BM) regulatory T (Treg) cells. Interaction of BM CD8+ T cells with Treg cells resulted in increased secretion of Wnt10b, a bone anabolic Wnt ligand. Mechanistically, Treg cells promoted the assembly of a NFAT1-SMAD3 transcription complex in CD8+ cells, which drove expression of Wnt10b. Reducing Treg cell numbers, or reconstitution of TCRβ-/- mice with CD8+ T cells from Wnt10b-/- mice, prevented butyrate-induced bone formation and bone mass acquisition. Thus, butyrate concentrations regulate bone anabolism via Treg cell-mediated regulation of CD8+ T cell Wnt10b production.
Keywords: Lactobacillus rhamnosus GG; NFAT; T cells; Wnt10b; bone formation; butyrate; microbiota; probiotics; regulatory T cells; short-chain fatty acids.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures





Comment in
-
Wnt signalling in the gut microbiota-bone axis.Nat Rev Rheumatol. 2019 Jan;15(1):4. doi: 10.1038/s41584-018-0139-9. Nat Rev Rheumatol. 2019. PMID: 30531853 No abstract available.
-
Of bugs, bones and butyrate.Nat Rev Immunol. 2019 Jan;19(1):4-5. doi: 10.1038/s41577-018-0104-5. Nat Rev Immunol. 2019. PMID: 30546108 No abstract available.
-
Make (No) Bones about Butyrate.Immunity. 2018 Dec 18;49(6):994-996. doi: 10.1016/j.immuni.2018.12.005. Immunity. 2018. PMID: 30566888
Similar articles
-
Parathyroid hormone-dependent bone formation requires butyrate production by intestinal microbiota.J Clin Invest. 2020 Apr 1;130(4):1767-1781. doi: 10.1172/JCI133473. J Clin Invest. 2020. PMID: 31917685 Free PMC article.
-
Lactobacillus rhamnosus GG supplementation modulates the gut microbiota to promote butyrate production, protecting against deoxynivalenol exposure in nude mice.Biochem Pharmacol. 2020 May;175:113868. doi: 10.1016/j.bcp.2020.113868. Epub 2020 Feb 20. Biochem Pharmacol. 2020. PMID: 32088259
-
T lymphocytes amplify the anabolic activity of parathyroid hormone through Wnt10b signaling.Cell Metab. 2009 Sep;10(3):229-40. doi: 10.1016/j.cmet.2009.07.010. Cell Metab. 2009. PMID: 19723499 Free PMC article.
-
Lactobacillus rhamnosus GG alleviates radiation-induced intestinal injury by modulating intestinal immunity and remodeling gut microbiota.Microbiol Res. 2024 Sep;286:127821. doi: 10.1016/j.micres.2024.127821. Epub 2024 Jun 25. Microbiol Res. 2024. PMID: 38941923
-
Limosilactobacillus reuteri and Lacticaseibacillus rhamnosus GG differentially affect gut microbes and metabolites in mice with Treg deficiency.Am J Physiol Gastrointest Liver Physiol. 2021 Jun 1;320(6):G969-G981. doi: 10.1152/ajpgi.00072.2021. Epub 2021 Mar 31. Am J Physiol Gastrointest Liver Physiol. 2021. PMID: 33787352 Free PMC article.
Cited by
-
Anaerostipes caccae CML199 enhances bone development and counteracts aging-induced bone loss through the butyrate-driven gut-bone axis: the chicken model.Microbiome. 2024 Oct 22;12(1):215. doi: 10.1186/s40168-024-01920-y. Microbiome. 2024. PMID: 39438898 Free PMC article.
-
Gut microbiota alterations in adolescent idiopathic scoliosis: a comparison study with healthy control and congenital scoliosis.Spine Deform. 2024 Oct 22. doi: 10.1007/s43390-024-00988-8. Online ahead of print. Spine Deform. 2024. PMID: 39438431
-
In-silico analysis predicts disruption of normal angiogenesis as a causative factor in osteoporosis pathogenesis.BMC Genom Data. 2024 Oct 8;25(1):85. doi: 10.1186/s12863-024-01269-z. BMC Genom Data. 2024. PMID: 39379846 Free PMC article.
-
The balance between helper T 17 and regulatory T cells in osteoimmunology and relevant research progress on bone tissue engineering.Immun Inflamm Dis. 2024 Sep;12(9):e70011. doi: 10.1002/iid3.70011. Immun Inflamm Dis. 2024. PMID: 39264247 Free PMC article. Review.
-
Advances on T cell immunity in bone remodeling and bone regeneration.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024 Aug 25;53(4):450-459. doi: 10.3724/zdxbyxb-2023-0619. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 39183057 Free PMC article. Review. Chinese, English.
References
-
- Almeida M, Han L, Bellido T, Manolagas SC, and Kousteni S (2005). Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J. Biol. Chem 280, 41342–41351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

