One Step Beyond: Design of Substrates Spanning Primed Positions of Zika Virus NS2B-NS3 Protease
- PMID: 30344911
- PMCID: PMC6187398
- DOI: 10.1021/acsmedchemlett.8b00316
One Step Beyond: Design of Substrates Spanning Primed Positions of Zika Virus NS2B-NS3 Protease
Abstract
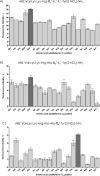
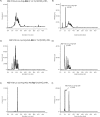
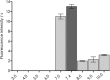
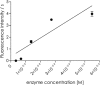
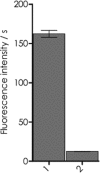
Although the mosquito-borne Zika virus was discovered in the late 1940s of the 20th century, for years it was neglected, as the disease in humans was rare and relatively mild. Viral NS2B-NS3 protease is essential for virus replication, and except for maturation of viral proteins, it also modulates the infection microenvironment to facilitate virus invasion. Here, we report the combinatorial chemistry approach for the synthesis of internally quenched substrates of the Zika virus NS2B-NS3 protease that were optimized in prime positions of the peptide chain. Final substrate ABZ-Val-Lys-Lys-Arg-Ala-Ala-Trp-Tyr(3-NO2)-NH2 displays an excellent kinetic parameter (k cat/K M reaching nearly 1.26 × 108 M-1 × s-1), which is over 10 times greater than previously reported (7.7 × 106 M-1 × s-1) substrate. Moreover, it was found to be selective over West Nile virus protease.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Probing the substrate specificity of the dengue virus type 2 NS3 serine protease by using internally quenched fluorescent peptides.Biochem J. 2006 Jul 1;397(1):203-11. doi: 10.1042/BJ20051767. Biochem J. 2006. PMID: 16489931 Free PMC article.
-
Novel tools to study West Nile virus NS3 protease activity.Bioorg Chem. 2023 Apr;133:106426. doi: 10.1016/j.bioorg.2023.106426. Epub 2023 Feb 15. Bioorg Chem. 2023. PMID: 36801793
-
The Structure of the Zika Virus Protease, NS2B/NS3pro.Adv Exp Med Biol. 2018;1062:131-145. doi: 10.1007/978-981-10-8727-1_10. Adv Exp Med Biol. 2018. PMID: 29845530 Review.
-
A FRET-based assay for the discovery of West Nile Virus NS2B-NS3 protease inhibitors.Bioorg Med Chem Lett. 2013 Sep 1;23(17):4848-50. doi: 10.1016/j.bmcl.2013.06.081. Epub 2013 Jul 4. Bioorg Med Chem Lett. 2013. PMID: 23886689
-
West Nile Virus NS2B/NS3 protease as an antiviral target.Curr Med Chem. 2008;15(27):2771-84. doi: 10.2174/092986708786242804. Curr Med Chem. 2008. PMID: 18991636 Review.
Cited by
-
The Inhibition of NS2B/NS3 Protease: A New Therapeutic Opportunity to Treat Dengue and Zika Virus Infection.Int J Mol Sci. 2024 Apr 16;25(8):4376. doi: 10.3390/ijms25084376. Int J Mol Sci. 2024. PMID: 38673962 Free PMC article. Review.
-
Mechanisms of Action for Small Molecules Revealed by Structural Biology in Drug Discovery.Int J Mol Sci. 2020 Jul 24;21(15):5262. doi: 10.3390/ijms21155262. Int J Mol Sci. 2020. PMID: 32722222 Free PMC article. Review.
-
A mutation-mediated evolutionary adaptation of Zika virus in mosquito and mammalian host.Proc Natl Acad Sci U S A. 2021 Oct 19;118(42):e2113015118. doi: 10.1073/pnas.2113015118. Proc Natl Acad Sci U S A. 2021. PMID: 34620704 Free PMC article.
References
-
- ICTV. Virus taxonomy. 2014.
-
- Pierson T. C., Diamond M. S.. Flaviviruses. In Fiels Virol, 6th ed.; Knipe D. M., Howley P. M., Eds.; Lippincott Williams & Wilkins: Philadelphia, 2013; pp 747–94.
-
- Leung D.; Schroder K.; White H.; Fang N. X.; Stoermer M. J.; Abbenante F.; Martin J. L.; Young P. R.; Fairlie D. P. Activity of recombinant dengue 2 virus NS3 protease in the presence of a truncated NS2B co-factor, small peptide substrates, and inhibitors. J. Biol. Chem. 2001, 276, 45762–771. 10.1074/jbc.M107360200. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

