Dendritic Cell Cancer Therapy: Vaccinating the Right Patient at the Right Time
- PMID: 30327656
- PMCID: PMC6174277
- DOI: 10.3389/fimmu.2018.02265
Dendritic Cell Cancer Therapy: Vaccinating the Right Patient at the Right Time
Abstract
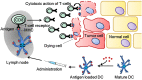
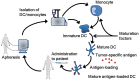
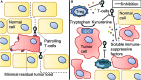
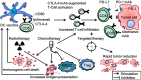
Immune checkpoint inhibitors propelled the field of oncology with clinical responses in many different tumor types. Superior overall survival over chemotherapy has been reported in various metastatic cancers. Furthermore, prolonged disease-free and overall survival have been reported in the adjuvant treatment of stage III melanoma. Unfortunately, a substantial portion of patients do not obtain a durable response. Therefore, additional strategies for the treatment of cancer are still warranted. One of the numerous options is dendritic cell vaccination, which employs the central role of dendritic cells in activating the innate and adaptive immune system. Over the years, dendritic cell vaccination was shown to be able to induce an immunologic response, to increase the number of tumor infiltrating lymphocytes and to provide overall survival benefit for at least a selection of patients in phase II studies. However, with the success of immune checkpoint inhibition in several malignancies and considering the plethora of other treatment modalities being developed, it is of utmost importance to delineate the position of dendritic cell therapy in the treatment landscape of cancer. In this review, we address some key questions regarding the integration of dendritic cell vaccination in future cancer treatment paradigms.
Keywords: adjuvant; cancer; checkpoint inhibitor; dendritic cell; immunotherapy; vaccination.
Figures
Similar articles
-
Dendritic Cells and Cancer Immunotherapy: The Adjuvant Effect.Int J Mol Sci. 2021 Nov 15;22(22):12339. doi: 10.3390/ijms222212339. Int J Mol Sci. 2021. PMID: 34830221 Free PMC article. Review.
-
Dendritic cell vaccination in melanoma patients: From promising results to future perspectives.Hum Vaccin Immunother. 2016 Oct 2;12(10):2523-2528. doi: 10.1080/21645515.2016.1197453. Epub 2016 Jun 20. Hum Vaccin Immunother. 2016. PMID: 27322496 Free PMC article.
-
In vivo cancer vaccination: Which dendritic cells to target and how?Cancer Treat Rev. 2018 Dec;71:88-101. doi: 10.1016/j.ctrv.2018.10.012. Epub 2018 Oct 25. Cancer Treat Rev. 2018. PMID: 30390423 Free PMC article. Review.
-
Immune adjuvants as critical guides directing immunity triggered by therapeutic cancer vaccines.Cytotherapy. 2014 Apr;16(4):427-39. doi: 10.1016/j.jcyt.2013.09.008. Epub 2013 Nov 23. Cytotherapy. 2014. PMID: 24280238 Review.
-
Dendritic Cells and Programmed Death-1 Blockade: A Joint Venture to Combat Cancer.Front Immunol. 2018 Mar 1;9:394. doi: 10.3389/fimmu.2018.00394. eCollection 2018. Front Immunol. 2018. PMID: 29599770 Free PMC article. Review.
Cited by
-
Combinational Pulsing of TAAs Enforces Dendritic Cell-Based Immunotherapy through T-Cell Proliferation and Interferon-γ Secretion in LLC1 Mouse Model.Cancers (Basel). 2024 Jan 18;16(2):409. doi: 10.3390/cancers16020409. Cancers (Basel). 2024. PMID: 38254898 Free PMC article.
-
Development of a Human Cytomegalovirus (HCMV)-Based Therapeutic Cancer Vaccine Uncovers a Previously Unsuspected Viral Block of MHC Class I Antigen Presentation.Front Immunol. 2019 Jul 30;10:1776. doi: 10.3389/fimmu.2019.01776. eCollection 2019. Front Immunol. 2019. PMID: 31417555 Free PMC article.
-
Chemo-immunotherapy improves long-term survival in a preclinical model of MMR-D-related cancer.J Immunother Cancer. 2019 Jan 10;7(1):8. doi: 10.1186/s40425-018-0476-x. J Immunother Cancer. 2019. PMID: 30630527 Free PMC article.
-
Resistance mechanisms in melanoma to immuneoncologic therapy with checkpoint inhibitors.Cancer Drug Resist. 2019 Sep 19;2(3):744-761. doi: 10.20517/cdr.2019.28. eCollection 2019. Cancer Drug Resist. 2019. PMID: 35582566 Free PMC article. Review.
-
Therapeutic Cancer Vaccination with Ex Vivo RNA-Transfected Dendritic Cells-An Update.Pharmaceutics. 2020 Jan 23;12(2):92. doi: 10.3390/pharmaceutics12020092. Pharmaceutics. 2020. PMID: 31979205 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

