Stabilized collagen matrix dressing improves wound macrophage function and epithelialization
- PMID: 30260708
- PMCID: PMC6338656
- DOI: 10.1096/fj.201800352R
Stabilized collagen matrix dressing improves wound macrophage function and epithelialization
Abstract
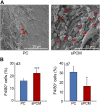
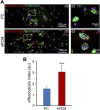
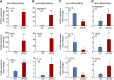
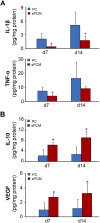
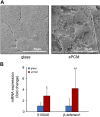
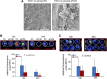
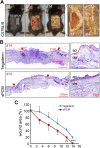
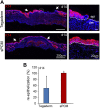
Decellularized matrices of biologic tissue have performed well as wound care dressings. Extracellular matrix-based dressings are subject to rapid degradation by excessive protease activity at the wound environment. Stabilized, acellular, equine pericardial collagen matrix (sPCM) wound care dressing is flexible cross-linked proteolytic enzyme degradation resistant. sPCM was structurally characterized utilizing scanning electron and atomic force microscopy. In murine excisional wounds, sPCM was effective in mounting an acute inflammatory response. Postwound inflammation resolved rapidly, as indicated by elevated levels of IL-10, arginase-1, and VEGF, and lowering of IL-1β and TNF-α. sPCM induced antimicrobial proteins S100A9 and β-defensin-1 in keratinocytes. Adherence of Pseudomonas aeruginosa and Staphylococcus aureus on sPCM pre-exposed to host immune cells in vivo was inhibited. Excisional wounds dressed with sPCM showed complete closure at d 14, while control wounds remained open. sPCM accelerated wound re-epithelialization. sPCM not only accelerated wound closure but also improved the quality of healing by increased collagen deposition and maturation. Thus, sPCM is capable of presenting scaffold functionality during the course of wound healing. In addition to inducing endogenous antimicrobial defense systems, the dressing itself has properties that minimize biofilm formation. It mounts robust inflammation, a process that rapidly resolves, making way for wound healing to advance.-El Masry, M. S., Chaffee, S., Das Ghatak, P., Mathew-Steiner, S. S., Das, A., Higuita-Castro, N., Roy, S., Anani, R. A., Sen, C. K. Stabilized collagen matrix dressing improves wound macrophage function and epithelialization.
Keywords: ECM; antimicrobial peptides; biofilm; cytokines; scaffold.
Conflict of interest statement
The authors thank Dr. B. Deng (Center for Electron Microscopy and Analysis College of Engineering, The Ohio State University) for her assistance with light microscopy studies on sPCM. This work was supported by U.S. National Institutes of Health Grants GM077185 and GM069589 (National Institute of General Medical Sciences), NR013898 and NR015676 (National Institute of Nursing Research), and DK076566 (National Institute of Diabetes and Digestive and Kidney Diseases). This work was supported, in part, by an unrestricted gift from Harbor MedTech (Irvine, CA, USA). The authors declare no conflicts of interest.
Figures

Similar articles
-
Neurotensin-loaded collagen dressings reduce inflammation and improve wound healing in diabetic mice.Biochim Biophys Acta. 2014 Jan;1842(1):32-43. doi: 10.1016/j.bbadis.2013.10.009. Epub 2013 Oct 23. Biochim Biophys Acta. 2014. PMID: 24161538
-
Inhibition of Pseudomonas aeruginosa biofilm formation on wound dressings.Wound Repair Regen. 2015 Nov-Dec;23(6):842-54. doi: 10.1111/wrr.12365. Epub 2015 Nov 4. Wound Repair Regen. 2015. PMID: 26342168 Free PMC article.
-
Novel murine model for delayed wound healing using a biological wound dressing with Pseudomonas aeruginosa biofilms.Microb Pathog. 2018 Sep;122:30-38. doi: 10.1016/j.micpath.2018.05.043. Epub 2018 May 26. Microb Pathog. 2018. PMID: 29842898
-
Cobalt-mediated multi-functional dressings promote bacteria-infected wound healing.Acta Biomater. 2019 Mar 1;86:465-479. doi: 10.1016/j.actbio.2018.12.048. Epub 2018 Dec 30. Acta Biomater. 2019. PMID: 30599244
-
Impact of a novel, antimicrobial dressing on in vivo, Pseudomonas aeruginosa wound biofilm: quantitative comparative analysis using a rabbit ear model.Wound Repair Regen. 2014 Nov-Dec;22(6):712-9. doi: 10.1111/wrr.12232. Epub 2015 Jan 8. Wound Repair Regen. 2014. PMID: 25230854
Cited by
-
The clinical efficacy of collagen dressing on chronic wounds: A meta-analysis of 11 randomized controlled trials.Front Surg. 2022 Aug 31;9:978407. doi: 10.3389/fsurg.2022.978407. eCollection 2022. Front Surg. 2022. PMID: 36117827 Free PMC article. Review.
-
Efficacy of Topical Application of Chum Salmon (Oncorhynchus keta) Skin-derived Collagen Extracts in Improving Oral Traumatic Ulcer Healing.Contemp Clin Dent. 2024 Apr-Jun;15(2):124-128. doi: 10.4103/ccd.ccd_544_22. Epub 2024 Jul 10. Contemp Clin Dent. 2024. PMID: 39206236 Free PMC article.
-
Filamentous bacteriophage delays healing of Pseudomonas-infected wounds.Cell Rep Med. 2022 Jun 21;3(6):100656. doi: 10.1016/j.xcrm.2022.100656. Cell Rep Med. 2022. PMID: 35732145 Free PMC article.
-
Exosome-Mediated Crosstalk between Keratinocytes and Macrophages in Cutaneous Wound Healing.ACS Nano. 2020 Oct 27;14(10):12732-12748. doi: 10.1021/acsnano.0c03064. Epub 2020 Sep 25. ACS Nano. 2020. PMID: 32931251 Free PMC article.
-
High resolution ultrasound imaging for repeated measure of wound tissue morphometry, biomechanics and hemodynamics under fetal, adult and diabetic conditions.PLoS One. 2020 Nov 23;15(11):e0241831. doi: 10.1371/journal.pone.0241831. eCollection 2020. PLoS One. 2020. PMID: 33227015 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

