Antimicrobial Potential of Silver Nanoparticles Synthesized Using Medicinal Herb Coptidis rhizome
- PMID: 30189681
- PMCID: PMC6225263
- DOI: 10.3390/molecules23092269
Antimicrobial Potential of Silver Nanoparticles Synthesized Using Medicinal Herb Coptidis rhizome
Abstract
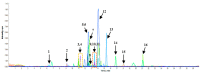
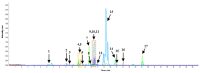
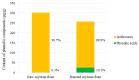
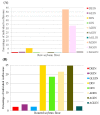
Coptidisrhizome contains several alkaloids that are bioactive agents of therapeutic value. We propose an eco-friendly method to synthesize biocompatible silver nanoparticles (AgNPs) using the aqueous extract of Coptidisrhizome. Silver ions were reduced to AgNPs using the aqueous extract of Coptidisrhizome, indicating that Coptidisrhizome can be used for the biosynthesis of AgNPs. The time and the concentration required for conversion of silver ions into AgNPs was optimized using UV-absorbance spectroscopy and inductively coupled plasma spectroscopy (ICP). Biosynthesized AgNPs showed a distinct UV-Visible absorption peak at 420 nm. ICP analysis showed that the time required for the completion of biosynthesis was around 20 min. Microscopic images showed that nanoparticles synthesized were of spherical shape and the average diameter of biosynthesized AgNPs was less than 30 nm. XRD analysis also confirmed the size of AgNps and revealed their crystalline nature. The interaction of AgNPs with phytochemicals present in Coptidisrhizome extract was observed in FTIR analysis. The antimicrobial property of AgNPs was evaluated using turbidity measurements. Coptidisrhizome-mediated biosynthesized AgNPs showed significant anti-bacterial activities against Escherichia coli and Staphylococcus aureus that are commonly involved in various types of infections, indicating their potential as an effective anti-bacterial agent.
Keywords: Coptidis rhizome; antibacterial; biosynthesis; silver nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Antimicrobial Potential of Silver Nanoparticles Synthesized Using Medicinal Herb Coptidis rhizome.Molecules. 2018 Sep 5;23(9):2268. doi: 10.3390/molecules23092268. Molecules. 2018. PMID: 30189672 Free PMC article.
-
Antimicrobial, Antioxidant, and Anticancer Activities of Biosynthesized Silver Nanoparticles Using Marine Algae Ecklonia cava.Nanomaterials (Basel). 2016 Dec 6;6(12):235. doi: 10.3390/nano6120235. Nanomaterials (Basel). 2016. PMID: 28335363 Free PMC article.
-
Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications.Artif Cells Nanomed Biotechnol. 2018 Sep;46(6):1163-1170. doi: 10.1080/21691401.2017.1362417. Epub 2017 Aug 8. Artif Cells Nanomed Biotechnol. 2018. PMID: 28784039
-
A review of microbes mediated biosynthesis of silver nanoparticles and their enhanced antimicrobial activities.Heliyon. 2024 Jun 4;10(11):e32333. doi: 10.1016/j.heliyon.2024.e32333. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38947433 Free PMC article. Review.
-
Plant Extract-Synthesized Silver Nanoparticles for Application in Dental Therapy.Pharmaceutics. 2022 Feb 8;14(2):380. doi: 10.3390/pharmaceutics14020380. Pharmaceutics. 2022. PMID: 35214112 Free PMC article. Review.
References
-
- Berk Z. Technology of Production of Edible Flours and Protein Products from Soybeans. Food and Agriculture Organization of the United Nations; Rome, Italy: 1992. p. 4.
-
- Liu K. Soybeans as Functional Foods and Ingredients. American Oil Chemists’ Society Press; Urbana, IL, USA: 2004. pp. 1–22.
LinkOut - more resources
Full Text Sources
Other Literature Sources

