Neutrophils Promote Amphiregulin Production in Intestinal Epithelial Cells through TGF-β and Contribute to Intestinal Homeostasis
- PMID: 30171165
- PMCID: PMC6179911
- DOI: 10.4049/jimmunol.1800003
Neutrophils Promote Amphiregulin Production in Intestinal Epithelial Cells through TGF-β and Contribute to Intestinal Homeostasis
Abstract
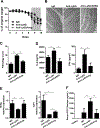
Neutrophils are the first responders to sites of inflammation when the intestinal epithelial barrier is breached and the gut microbiota invade. Despite current efforts in understanding the role of neutrophils in intestinal homeostasis, the complex interactions between neutrophils and intestinal epithelial cells (IECs) is still not well characterized. In this study, we demonstrated that neutrophils enhanced production of amphiregulin (AREG), a member of the EGFR ligand family, by IECs, which promoted IEC barrier function and tissue repair. Depletion of neutrophils resulted in more severe colitis in mice because of decreased AREG production by IECs upon dextran sodium sulfate (DSS) insult. Administration of AREG restored epithelial barrier function and ameliorated colitis. Furthermore, neutrophil-derived TGF-β promoted AREG production by IECs. Mechanistically, TGF-β activated MEK1/2 signaling, and inhibition of MEK1/2 abrogated TGF-β-induced AREG production by IECs. Collectively, these findings reveal that neutrophils play an important role in the maintenance of IEC barrier function and homeostasis.
Copyright © 2018 by The American Association of Immunologists, Inc.
Conflict of interest statement
Figures

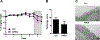
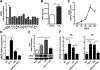
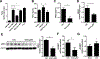
Similar articles
-
IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions.Proc Natl Acad Sci U S A. 2015 Aug 25;112(34):10762-7. doi: 10.1073/pnas.1509070112. Epub 2015 Aug 4. Proc Natl Acad Sci U S A. 2015. PMID: 26243875 Free PMC article.
-
Th17 Cell-Derived Amphiregulin Promotes Colitis-Associated Intestinal Fibrosis Through Activation of mTOR and MEK in Intestinal Myofibroblasts.Gastroenterology. 2023 Jan;164(1):89-102. doi: 10.1053/j.gastro.2022.09.006. Epub 2022 Sep 13. Gastroenterology. 2023. PMID: 36113570 Free PMC article.
-
Neutrophil-derived PAD4 induces citrullination of CKMT1 exacerbates mucosal inflammation in inflammatory bowel disease.Cell Mol Immunol. 2024 Jun;21(6):620-633. doi: 10.1038/s41423-024-01158-6. Epub 2024 May 8. Cell Mol Immunol. 2024. PMID: 38720063
-
TGF-β in inflammatory bowel disease: a key regulator of immune cells, epithelium, and the intestinal microbiota.J Gastroenterol. 2017 Jul;52(7):777-787. doi: 10.1007/s00535-017-1350-1. Epub 2017 May 22. J Gastroenterol. 2017. PMID: 28534191 Review.
-
Amphiregulin in infectious diseases: Role, mechanism, and potential therapeutic targets.Microb Pathog. 2024 Jan;186:106463. doi: 10.1016/j.micpath.2023.106463. Epub 2023 Nov 28. Microb Pathog. 2024. PMID: 38036111 Review.
Cited by
-
An intercrypt subpopulation of goblet cells is essential for colonic mucus barrier function.Science. 2021 Apr 16;372(6539):eabb1590. doi: 10.1126/science.abb1590. Science. 2021. PMID: 33859001 Free PMC article.
-
Paneth Cell-Derived Lysozyme Defines the Composition of Mucolytic Microbiota and the Inflammatory Tone of the Intestine.Immunity. 2020 Aug 18;53(2):398-416.e8. doi: 10.1016/j.immuni.2020.07.010. Immunity. 2020. PMID: 32814028 Free PMC article.
-
Identification of a prototype human gut Bifidobacterium longum subsp. longum strain based on comparative and functional genomic approaches.Front Microbiol. 2023 Feb 8;14:1130592. doi: 10.3389/fmicb.2023.1130592. eCollection 2023. Front Microbiol. 2023. PMID: 36846784 Free PMC article.
-
A critical role of AREG for bleomycin-induced skin fibrosis.Cell Biosci. 2021 Feb 23;11(1):40. doi: 10.1186/s13578-021-00553-0. Cell Biosci. 2021. PMID: 33622407 Free PMC article.
-
Intestinal Mucosal Wound Healing and Barrier Integrity in IBD-Crosstalk and Trafficking of Cellular Players.Front Med (Lausanne). 2021 Mar 23;8:643973. doi: 10.3389/fmed.2021.643973. eCollection 2021. Front Med (Lausanne). 2021. PMID: 33834033 Free PMC article. Review.
References
-
- Peterson LW, and Artis D. 2014. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14: 141–153. - PubMed
-
- van der Flier LG, and Clevers H. 2009. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71: 241–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

