Changes in proteome solubility indicate widespread proteostatic disruption in mouse models of neurodegenerative disease
- PMID: 30140941
- PMCID: PMC6411038
- DOI: 10.1007/s00401-018-1895-y
Changes in proteome solubility indicate widespread proteostatic disruption in mouse models of neurodegenerative disease
Abstract
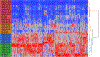
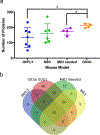
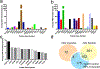
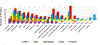
The deposition of pathologic misfolded proteins in neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, frontotemporal dementia and amyotrophic lateral sclerosis is hypothesized to burden protein homeostatic (proteostatic) machinery, potentially leading to insufficient capacity to maintain the proteome. This hypothesis has been supported by previous work in our laboratory, as evidenced by the perturbation of cytosolic protein solubility in response to amyloid plaques in a mouse model of Alzheimer's amyloidosis. In the current study, we demonstrate changes in proteome solubility are a common pathology to mouse models of neurodegenerative disease. Pathological accumulations of misfolded tau, α-synuclein and mutant superoxide dismutase 1 in CNS tissues of transgenic mice were associated with changes in the solubility of hundreds of CNS proteins in each model. We observed that changes in proteome solubility were progressive and, using the rTg4510 model of inducible tau pathology, demonstrated that these changes were dependent upon sustained expression of the primary pathologic protein. In all of the models examined, changes in proteome solubility were robust, easily detected, and provided a sensitive indicator of proteostatic disruption. Interestingly, a subset of the proteins that display a shift towards insolubility were common between these different models, suggesting that a specific subset of the proteome is vulnerable to proteostatic disruption. Overall, our data suggest that neurodegenerative proteinopathies modeled in mice impose a burden on the proteostatic network that diminishes the ability of neural cells to prevent aberrant conformational changes that alter the solubility of hundreds of abundant cellular proteins.
Keywords: Neurodegeneration; Protein misfolding; Proteinopathy; Proteomics; Proteostasis.
Conflict of interest statement
Figures



Similar articles
-
Exploring the Role of Aggregated Proteomes in the Pathogenesis of Alzheimer's Disease.Curr Protein Pept Sci. 2020;21(12):1164-1173. doi: 10.2174/1389203721666200921152246. Curr Protein Pept Sci. 2020. PMID: 32957903 Review.
-
Molecular and functional signatures in a novel Alzheimer's disease mouse model assessed by quantitative proteomics.Mol Neurodegener. 2018 Jan 16;13(1):2. doi: 10.1186/s13024-017-0234-4. Mol Neurodegener. 2018. PMID: 29338754 Free PMC article.
-
Differential induction of mutant SOD1 misfolding and aggregation by tau and α-synuclein pathology.Mol Neurodegener. 2018 May 18;13(1):23. doi: 10.1186/s13024-018-0253-9. Mol Neurodegener. 2018. PMID: 29776378 Free PMC article.
-
Tau reduction in the presence of amyloid-β prevents tau pathology and neuronal death in vivo.Brain. 2018 Jul 1;141(7):2194-2212. doi: 10.1093/brain/awy117. Brain. 2018. PMID: 29733334 Free PMC article.
-
Amyloid-induced neurofibrillary tangle formation in Alzheimer's disease: insight from transgenic mouse and tissue-culture models.Int J Dev Neurosci. 2004 Nov;22(7):453-65. doi: 10.1016/j.ijdevneu.2004.07.013. Int J Dev Neurosci. 2004. PMID: 15465275 Review.
Cited by
-
Shift of the insoluble content of the proteome in the aging mouse brain.Proc Natl Acad Sci U S A. 2023 Nov 7;120(45):e2310057120. doi: 10.1073/pnas.2310057120. Epub 2023 Oct 31. Proc Natl Acad Sci U S A. 2023. PMID: 37906643 Free PMC article.
-
Diversity in Aβ deposit morphology and secondary proteome insolubility across models of Alzheimer-type amyloidosis.Acta Neuropathol Commun. 2020 Apr 6;8(1):43. doi: 10.1186/s40478-020-00911-y. Acta Neuropathol Commun. 2020. PMID: 32252825 Free PMC article.
-
Myelin in Alzheimer's disease: culprit or bystander?Acta Neuropathol Commun. 2023 Mar 31;11(1):56. doi: 10.1186/s40478-023-01554-5. Acta Neuropathol Commun. 2023. PMID: 37004127 Free PMC article. Review.
-
Hsp90 and Its Co-Chaperones in Neurodegenerative Diseases.Int J Mol Sci. 2019 Oct 9;20(20):4976. doi: 10.3390/ijms20204976. Int J Mol Sci. 2019. PMID: 31600883 Free PMC article. Review.
-
Non-Proteasomal UbL-UbA Family of Proteins in Neurodegeneration.Int J Mol Sci. 2019 Apr 17;20(8):1893. doi: 10.3390/ijms20081893. Int J Mol Sci. 2019. PMID: 30999567 Free PMC article. Review.
References
-
- Ayyadevara S, Balasubramaniam M, Parcon PA, Barger SW, Griffin WST, Alla R, Tackett AJ, Mackintosh SG, Petricoin E, Zhou W, Shmookler Reis RJ (2016) Proteins that mediate protein aggregation and cytotoxicity distinguish Alzheimer’s hippocampus from normal controls. Aging Cell 15:924–939. doi: 10.1111/acel.12501 - DOI - PMC - PubMed
-
- Bai B, Hales CM, Chen P, Gozal Y, Dammer EB, Fritz JJ (2013) U1 small nuclear ribonucleoprotein complex and RNA splicing alterations in Alzheimer ‘ s disease. Proc Natl Acad Sci U S A 110:16562–16567. doi: 10.1073/pnas.1310249110/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1310249110 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

