Evaluation of platelet lysate as a substitute for FBS in explant and enzymatic isolation methods of human umbilical cord MSCs
- PMID: 30127445
- PMCID: PMC6102222
- DOI: 10.1038/s41598-018-30772-4
Evaluation of platelet lysate as a substitute for FBS in explant and enzymatic isolation methods of human umbilical cord MSCs
Abstract
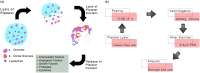
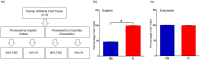
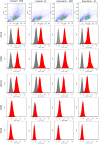
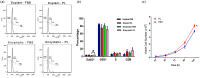
Mesenchymal stem cells (MSCs) have immense potential for cell-based therapy of acute and chronic pathological conditions. MSC transplantation for cell-based therapy requires a substantial number of cells in the range of 0.5-2.5 × 106 cells/kg body weight of an individual. A prolific source of MSCs followed by in vitro propagation is therefore an absolute prerequisite for clinical applications. Umbilical cord tissue (UCT) is an abundantly available prolific source of MSC that are fetal in nature and have higher potential for ex-vivo expansion. However, the ex-vivo expansion of MSCs using a xenogeneic supplement such as fetal bovine serum (FBS) carries the risk of transmission of zoonotic infections and immunological reactions. We used platelet lysate (PL) as a xeno-free, allogeneic replacement for FBS and compared the biological and functional characteristics of MSC processed and expanded with PL and FBS by explant and enzymatic method. UCT-MSCs expanded using PL displayed typical immunophenotype, plasticity, immunomodulatory property and chromosomal stability. PL supplementation also showed 2-fold increase in MSC yield from explant culture with improved immunomodulatory activity as compared to enzymatically dissociated cultures. In conclusion, PL from expired platelets is a viable alternative to FBS for generating clinically relevant numbers of MSC from explant cultures over enzymatic method.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Effect of allogeneic platelet lysate on equine bone marrow derived mesenchymal stem cell characteristics, including immunogenic and immunomodulatory gene expression profile.Vet Immunol Immunopathol. 2019 Nov;217:109944. doi: 10.1016/j.vetimm.2019.109944. Epub 2019 Sep 21. Vet Immunol Immunopathol. 2019. PMID: 31563725
-
Human platelet lysate in mesenchymal stromal cell expansion according to a GMP grade protocol: a cell factory experience.Stem Cell Res Ther. 2018 May 2;9(1):124. doi: 10.1186/s13287-018-0863-8. Stem Cell Res Ther. 2018. PMID: 29720245 Free PMC article.
-
Potency testing of mesenchymal stromal cell growth expanded in human platelet lysate from different human tissues.Stem Cell Res Ther. 2016 Aug 25;7(1):122. doi: 10.1186/s13287-016-0383-3. Stem Cell Res Ther. 2016. PMID: 27557940 Free PMC article.
-
Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: present and future.Stem Cell Res Ther. 2016 Jul 13;7(1):93. doi: 10.1186/s13287-016-0352-x. Stem Cell Res Ther. 2016. PMID: 27411942 Free PMC article. Review.
-
Preparation, quality criteria, and properties of human blood platelet lysate supplements for ex vivo stem cell expansion.N Biotechnol. 2015 Jan 25;32(1):199-211. doi: 10.1016/j.nbt.2014.06.001. Epub 2014 Jun 11. N Biotechnol. 2015. PMID: 24929129 Free PMC article. Review.
Cited by
-
Use of flow cytometry method to detect contaminations of platelet suspensions.World J Microbiol Biotechnol. 2024 May 30;40(7):222. doi: 10.1007/s11274-024-04030-x. World J Microbiol Biotechnol. 2024. PMID: 38811387 Free PMC article.
-
Differentiation of placenta-derived MSCs cultured in human platelet lysate: a xenofree supplement.3 Biotech. 2024 Apr;14(4):116. doi: 10.1007/s13205-024-03966-z. Epub 2024 Mar 22. 3 Biotech. 2024. PMID: 38524240
-
An optimized procedure for preparation of conditioned medium from Wharton's jelly mesenchymal stromal cells isolated from umbilical cord.Front Mol Biosci. 2023 Oct 2;10:1273814. doi: 10.3389/fmolb.2023.1273814. eCollection 2023. Front Mol Biosci. 2023. PMID: 37854039 Free PMC article.
-
Bioreactors, scaffolds and microcarriers and in vitro meat production-current obstacles and potential solutions.Front Nutr. 2023 Sep 6;10:1225233. doi: 10.3389/fnut.2023.1225233. eCollection 2023. Front Nutr. 2023. PMID: 37743926 Free PMC article. Review.
-
Expanding applications of allogeneic platelets, platelet lysates, and platelet extracellular vesicles in cell therapy, regenerative medicine, and targeted drug delivery.J Biomed Sci. 2023 Sep 14;30(1):79. doi: 10.1186/s12929-023-00972-w. J Biomed Sci. 2023. PMID: 37704991 Free PMC article. Review.
References
-
- Hamidian Jahromi, S., Estrada, C., Li, Y., Cheng, E. & Davies, J. E. Human Umbilical Cord Perivascular Cells and Human Bone Marrow Mesenchymal Stromal Cells Transplanted Intramuscularly Respond to a Distant Source of Inflammation. Stem cells and development, 10.1089/scd.2017.0248 (2018). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

