PIP2 depletion promotes TRPV4 channel activity in mouse brain capillary endothelial cells
- PMID: 30084828
- PMCID: PMC6117155
- DOI: 10.7554/eLife.38689
PIP2 depletion promotes TRPV4 channel activity in mouse brain capillary endothelial cells
Abstract
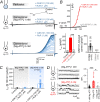
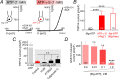
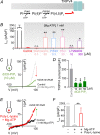
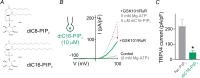
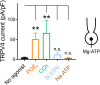
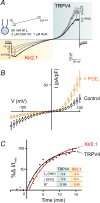
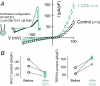
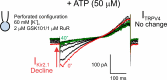
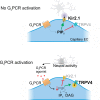
We recently reported that the inward-rectifier Kir2.1 channel in brain capillary endothelial cells (cECs) plays a major role in neurovascular coupling (NVC) by mediating a neuronal activity-dependent, propagating vasodilatory (hyperpolarizing) signal. We further demonstrated that Kir2.1 activity is suppressed by depletion of plasma membrane phosphatidylinositol 4,5-bisphosphate (PIP2). Whether cECs express depolarizing channels that intersect with Kir2.1-mediated signaling remains unknown. Here, we report that Ca2+/Na+-permeable TRPV4 (transient receptor potential vanilloid 4) channels are expressed in cECs and are tonically inhibited by PIP2. We further demonstrate that depletion of PIP2 by agonists, including putative NVC mediators, that promote PIP2 hydrolysis by signaling through Gq-protein-coupled receptors (GqPCRs) caused simultaneous disinhibition of TRPV4 channels and suppression of Kir2.1 channels. These findings collectively support the concept that GqPCR activation functions as a molecular switch to favor capillary TRPV4 activity over Kir2.1 signaling, an observation with potentially profound significance for the control of cerebral blood flow.
Keywords: GPCR; GqPCR; Kir2.1; PIP2; TRPV4; brain capillaries; molecular biophysics; mouse; neuroscience; neurovascular coupling; phosphoinositides; structural biology.
© 2018, Harraz et al.
Conflict of interest statement
OH, TL, DH, MN No competing interests declared
Figures






Similar articles
-
Endothelial GqPCR activity controls capillary electrical signaling and brain blood flow through PIP2 depletion.Proc Natl Acad Sci U S A. 2018 Apr 10;115(15):E3569-E3577. doi: 10.1073/pnas.1800201115. Epub 2018 Mar 26. Proc Natl Acad Sci U S A. 2018. PMID: 29581272 Free PMC article.
-
Inward rectifier potassium (Kir2.1) channels as end-stage boosters of endothelium-dependent vasodilators.J Physiol. 2016 Jun 15;594(12):3271-85. doi: 10.1113/JP271652. Epub 2016 Mar 4. J Physiol. 2016. PMID: 26840527 Free PMC article.
-
TRPV4 (Transient Receptor Potential Vanilloid 4) Channel-Dependent Negative Feedback Mechanism Regulates Gq Protein-Coupled Receptor-Induced Vasoconstriction.Arterioscler Thromb Vasc Biol. 2018 Mar;38(3):542-554. doi: 10.1161/ATVBAHA.117.310038. Epub 2018 Jan 4. Arterioscler Thromb Vasc Biol. 2018. PMID: 29301784 Free PMC article.
-
Endothelial signaling at the core of neurovascular coupling: The emerging role of endothelial inward-rectifier K+ (Kir2.1) channels and N-methyl-d-aspartate receptors in the regulation of cerebral blood flow.Int J Biochem Cell Biol. 2021 Jun;135:105983. doi: 10.1016/j.biocel.2021.105983. Epub 2021 Apr 21. Int J Biochem Cell Biol. 2021. PMID: 33894355 Review.
-
Endothelial TRPV4 channels and vasodilator reactivity.Curr Top Membr. 2020;85:89-117. doi: 10.1016/bs.ctm.2020.01.007. Epub 2020 Feb 12. Curr Top Membr. 2020. PMID: 32402646 Free PMC article. Review.
Cited by
-
Arachidonic Acid Evokes an Increase in Intracellular Ca2+ Concentration and Nitric Oxide Production in Endothelial Cells from Human Brain Microcirculation.Cells. 2019 Jul 9;8(7):689. doi: 10.3390/cells8070689. Cells. 2019. PMID: 31323976 Free PMC article.
-
Antagonism of TRPV4 channels partially reduces mechanotransduction in rat skeletal muscle afferents.J Physiol. 2023 Apr;601(8):1407-1424. doi: 10.1113/JP284026. Epub 2023 Mar 16. J Physiol. 2023. PMID: 36869605 Free PMC article.
-
Piezo1 Is a Mechanosensor Channel in Central Nervous System Capillaries.Circ Res. 2022 May 13;130(10):1531-1546. doi: 10.1161/CIRCRESAHA.122.320827. Epub 2022 Apr 6. Circ Res. 2022. PMID: 35382561 Free PMC article.
-
Augmented astrocyte microdomain Ca2+ dynamics and parenchymal arteriole tone in angiotensin II-infused hypertensive mice.Glia. 2019 Mar;67(3):551-565. doi: 10.1002/glia.23564. Epub 2018 Dec 2. Glia. 2019. PMID: 30506941 Free PMC article.
-
Transient Receptor Potential Channels and Endothelial Cell Calcium Signaling.Compr Physiol. 2019 Jun 12;9(3):1249-1277. doi: 10.1002/cphy.c180034. Compr Physiol. 2019. PMID: 31187891 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- Grant agreement No 666881 SVDs@target/European Union's Horizon 2020 Research and Innovation Programme/International
- R01-HL-131181/NH/NIH HHS/United States
- R01 HL121706/HL/NHLBI NIH HHS/United States
- P30-GM-103498 to the COBRE imaging facility at UVM College of Medicine/NH/NIH HHS/United States
- P01 HL095488/HL/NHLBI NIH HHS/United States
- P30 GM103498/GM/NIGMS NIH HHS/United States
- Postdoctoral fellowship 17POST33650030/American Heart Association/International
- R37-DK-053832/NH/NIH HHS/United States
- R37 DK053832/DK/NIDDK NIH HHS/United States
- R01-HL-121706/NH/NIH HHS/United States
- P20 GM103644/GM/NIGMS NIH HHS/United States
- P01-HL-095488/NH/NIH HHS/United States
- 4P20 GM103644/4-5 to the Vermont Center on Behavior and Health/NH/NIH HHS/United States
- R01 HL131181/HL/NHLBI NIH HHS/United States
- Scientist Development Grant 17SDG33670237/American Heart Association/International
- 7UM-HL-1207704/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

