Ductular Reaction in Liver Diseases: Pathological Mechanisms and Translational Significances
- PMID: 30070383
- PMCID: PMC6324973
- DOI: 10.1002/hep.30150
Ductular Reaction in Liver Diseases: Pathological Mechanisms and Translational Significances
Erratum in
-
Correction.Hepatology. 2019 Sep;70(3):1089. doi: 10.1002/hep.30878. Epub 2019 Aug 5. Hepatology. 2019. PMID: 31472030 No abstract available.
Abstract
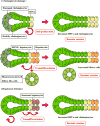
Ductular reaction (DR) is characterized by the proliferation of reactive bile ducts induced by liver injuries. DR is pathologically recognized as bile duct hyperplasia and is commonly observed in biliary disorders. It can also be identified in various liver disorders including nonalcoholic fatty liver disease. DR is associated with liver fibrosis and damage, and the extent of DR parallels to patient mortality. DR raises scientific interests because it is associated with transdifferentiation of liver cells and may play an important role in hepatic regeneration. The origin of active cells during DR can be cholangiocytes, hepatocytes, or hepatic progenitor cells, and associated signaling pathways could differ depending on the specific liver injury or animal models used in the study. Although further studies are needed to elucidate detailed mechanisms and the functional roles in liver diseases, DR can be a therapeutic target to inhibit liver fibrosis and to promote liver regeneration. This review summarizes previous studies of DR identified in patients and animal models as well as currently understood mechanisms of DR.
© 2018 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

Comment in
-
Letter to the Editor: Ductular Reaction in Acute Onset Autoimmune Hepatitis.Hepatology. 2019 Aug;70(2):756-757. doi: 10.1002/hep.30621. Hepatology. 2019. PMID: 30901095 No abstract available.
-
Reply.Hepatology. 2019 Aug;70(2):757. doi: 10.1002/hep.30619. Hepatology. 2019. PMID: 30912851 No abstract available.
Similar articles
-
[Clinical significance and correlation of ductular reaction in hepatobiliary diseases].Zhonghua Gan Zang Bing Za Zhi. 2018 Aug 20;26(8):637-640. doi: 10.3760/cma.j.issn.1007-3418.2018.08.017. Zhonghua Gan Zang Bing Za Zhi. 2018. PMID: 30317801 Chinese.
-
Unraveling the complexities of fibrosis and ductular reaction in liver disease: pathogenesis, mechanisms, and therapeutic insights.Am J Physiol Cell Physiol. 2024 Mar 1;326(3):C698-C706. doi: 10.1152/ajpcell.00486.2023. Epub 2023 Dec 18. Am J Physiol Cell Physiol. 2024. PMID: 38105754 Review.
-
Three-dimensional analysis of ductular reactions and their correlation with liver regeneration and fibrosis.Virchows Arch. 2024 May;484(5):753-763. doi: 10.1007/s00428-023-03641-3. Epub 2023 Sep 14. Virchows Arch. 2024. PMID: 37704824
-
Contributions of hepatocytes and bile ductular cells in ductular reactions and remodeling of the biliary system after chronic liver injury.Am J Pathol. 2014 Nov;184(11):3001-12. doi: 10.1016/j.ajpath.2014.07.005. Epub 2014 Sep 2. Am J Pathol. 2014. PMID: 25193593
-
[Hepatic neoductules].Verh Dtsch Ges Pathol. 1995;79:36-46. Verh Dtsch Ges Pathol. 1995. PMID: 8600693 Review. German.
Cited by
-
[Changes of YAP activity at the early stage of nonalcoholic steatohepatitis and its spatiotemporal relationship with ductular reaction in mice].Nan Fang Yi Ke Da Xue Xue Bao. 2022 Sep 20;42(9):1324-1334. doi: 10.12122/j.issn.1673-4254.2022.09.08. Nan Fang Yi Ke Da Xue Xue Bao. 2022. PMID: 36210705 Free PMC article. Chinese.
-
Ductular Reaction and Liver Regeneration: Fulfilling the Prophecy of Prometheus!Cell Mol Gastroenterol Hepatol. 2023;15(3):806-808. doi: 10.1016/j.jcmgh.2022.11.007. Epub 2022 Nov 24. Cell Mol Gastroenterol Hepatol. 2023. PMID: 36436755 Free PMC article. No abstract available.
-
Mallory-Denk bodies and hepatocellular senescence: a causal relationship?Virchows Arch. 2024 Apr;484(4):637-644. doi: 10.1007/s00428-024-03748-1. Epub 2024 Jan 30. Virchows Arch. 2024. PMID: 38289501 Free PMC article.
-
Hepatocytes demarcated by EphB2 contribute to the progression of nonalcoholic steatohepatitis.Sci Transl Med. 2023 Feb 8;15(682):eadc9653. doi: 10.1126/scitranslmed.adc9653. Epub 2023 Feb 8. Sci Transl Med. 2023. PMID: 36753562 Free PMC article.
-
Bioinformatics analysis identifies heparan sulfate proteoglycans acting as different progress subtypes of biliary atresia.Front Pediatr. 2023 Feb 2;11:1065521. doi: 10.3389/fped.2023.1065521. eCollection 2023. Front Pediatr. 2023. PMID: 36816373 Free PMC article.
References
-
- Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, Brunt EM, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–1745. - PubMed
-
- Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, Irvine K, et al. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59:1393–1405. - PubMed
-
- Gouw AS, van den Heuvel MC, Boot M, Slooff MJ, Poppema S, de Jong KP. Dynamics of the vascular profile of the finer branches of the biliary tree in normal and diseased human livers. J Hepatol. 2006;45:393–400. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 DK108959/DK/NIDDK NIH HHS/United States
- R21 AA025997/AA/NIAAA NIH HHS/United States
- R01 DK062975/DK/NIDDK NIH HHS/United States
- 5I01BX000574/VA Research Career Scientist Award/International
- VA Merit Award/International
- R01 DK058411/DK/NIDDK NIH HHS/United States
- R01 DK110035/DK/NIDDK NIH HHS/United States
- R01 DK107310/DK/NIDDK NIH HHS/United States
- 1I01BX003031/VA Research Career Scientist Award/International
- I01 BX000574/BX/BLRD VA/United States
- 1I01BX001724/VA Research Career Scientist Award/International
- Central Texas Veterans Health Care System/International
- R01 DK115184/DK/NIDDK NIH HHS/United States
- Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology/International
- I01 BX002192/BX/BLRD VA/United States
- I01 BX001724/BX/BLRD VA/United States
- R01 DK076898/DK/NIDDK NIH HHS/United States
- I01 BX003031/BX/BLRD VA/United States
- 5I01BX002192/VA Research Career Scientist Award/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

