AU040320 deficiency leads to disruption of acrosome biogenesis and infertility in homozygous mutant mice
- PMID: 29991750
- PMCID: PMC6039479
- DOI: 10.1038/s41598-018-28666-6
AU040320 deficiency leads to disruption of acrosome biogenesis and infertility in homozygous mutant mice
Abstract
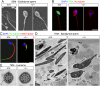
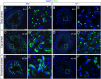
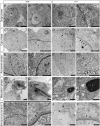
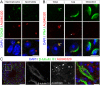
Study of knockout (KO) mice has helped understand the link between many genes/proteins and human diseases. Identification of infertile KO mice provides valuable tools to characterize the molecular mechanisms underlying gamete formation. The KIAA0319L gene has been described to have a putative association with dyslexia; surprisingly, we observed that homozygous KO males for AU040320, KIAA0319L ortholog, are infertile and present a globozoospermia-like phenotype. Mutant spermatozoa are mostly immotile and display a malformed roundish head with no acrosome. In round spermatids, proacrosomal vesicles accumulate close to the acroplaxome but fail to coalesce into a single acrosomal vesicle. In wild-type mice AU040320 localises to the trans-Golgi-Network of germ cells but cannot be detected in mature acrosomes. Our results suggest AU040320 may be necessary for the normal formation of proacrosomal vesicles or the recruitment of cargo proteins required for downstream events leading to acrosomal fusion. Mutations in KIAA0319L could lead to human infertility; we screened for KIAA0319L mutations in a selected cohort of globozoospermia patients in which no genetic abnormalities have been previously identified, but detected no pathogenic changes in this particular cohort.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Loss of the Na+/H+ exchanger NHE8 causes male infertility in mice by disrupting acrosome formation.J Biol Chem. 2017 Jun 30;292(26):10845-10854. doi: 10.1074/jbc.M117.784108. Epub 2017 May 5. J Biol Chem. 2017. PMID: 28476888 Free PMC article.
-
Globozoospermia and lack of acrosome formation in GM130-deficient mice.Cell Death Dis. 2017 Jan 5;8(1):e2532. doi: 10.1038/cddis.2016.414. Cell Death Dis. 2017. PMID: 28055014 Free PMC article.
-
Absence of Dpy19l2, a new inner nuclear membrane protein, causes globozoospermia in mice by preventing the anchoring of the acrosome to the nucleus.Development. 2012 Aug;139(16):2955-65. doi: 10.1242/dev.077982. Epub 2012 Jul 4. Development. 2012. PMID: 22764053
-
Human globozoospermia-related genes and their role in acrosome biogenesis.WIREs Mech Dis. 2023 Mar;15(2):e1589. doi: 10.1002/wsbm.1589. Epub 2022 Dec 9. WIREs Mech Dis. 2023. PMID: 36493758 Review.
-
Molecular cytogenetic and genetic aspects of globozoospermia: a review.Andrologia. 2013 Feb;45(1):1-9. doi: 10.1111/j.1439-0272.2012.01308.x. Epub 2012 May 10. Andrologia. 2013. PMID: 22571172 Review.
Cited by
-
Sperm Differentiation: The Role of Trafficking of Proteins.Int J Mol Sci. 2020 May 24;21(10):3702. doi: 10.3390/ijms21103702. Int J Mol Sci. 2020. PMID: 32456358 Free PMC article. Review.
-
A Non-Synonymous Point Mutation in a WD-40 Domain Repeat of EML5 Leads to Decreased Bovine Sperm Quality and Fertility.Front Cell Dev Biol. 2022 Apr 5;10:872740. doi: 10.3389/fcell.2022.872740. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35478957 Free PMC article.
-
FAM71F1 binds to RAB2A and RAB2B and is essential for acrosome formation and male fertility in mice.Development. 2021 Nov 1;148(21):dev199644. doi: 10.1242/dev.199644. Epub 2021 Oct 29. Development. 2021. PMID: 34714330 Free PMC article.
-
The deubiquitinase cofactor UAF1 interacts with USP1 and plays an essential role in spermiogenesis.iScience. 2024 Mar 11;27(4):109456. doi: 10.1016/j.isci.2024.109456. eCollection 2024 Apr 19. iScience. 2024. PMID: 38591005 Free PMC article.
-
Novel HYDIN variants associated with male infertility in two Chinese families.Front Endocrinol (Lausanne). 2023 Jan 18;14:1118841. doi: 10.3389/fendo.2023.1118841. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36742411 Free PMC article.
References
-
- Hess, R. A. & Renato de Franca, L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol.636, 1–15, 10.1007/978-0-387-09597-4_1 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

