TUG1 promotes prostate cancer progression by acting as a ceRNA of miR-26a
- PMID: 29967294
- PMCID: PMC6167503
- DOI: 10.1042/BSR20180677
TUG1 promotes prostate cancer progression by acting as a ceRNA of miR-26a
Abstract
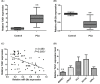
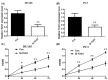
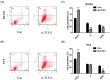
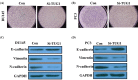
Previous studies have demonstrated that taurine-upregulated gene 1 (TUG1) was aberrantly expressed and involved in multiple types of cancer; however, the expression profile and potential role of TUG1 in prostate cancer (PCa) remains unclear. The aim of the present study was to evaluate the expression and function of TUG1 in PCa. In the present study, we analyzed TUG1 expression levels of PCa patients in tumor and adjacent normal tissue by real-time quantitative PCR. Knockdown of TUG1 by RNAi was performed to explore its roles in cell proliferation, migration, and invasion. Here we report, for the first time, that TUG1 promotes tumor cell migration, invasion, and proliferation in PCa by working in key aspects of biological behaviors. TUG1 could negatively regulate the expression of miR-26a in PCa cells. The bioinformatics prediction revealed putative miR-26a-binding sites within TUG1 transcripts. In conclusion, our study suggests that long non-coding RNA (lncRNA) TUG1 acts as a functional oncogene in PCa development.
Keywords: EMT; LncRNA; PCa; TUG1; metastasis; miR-26a.
© 2018 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
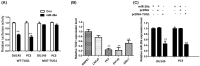
Similar articles
-
Long non-coding RNA HOTTIP promotes prostate cancer cells proliferation and migration by sponging miR-216a-5p.Biosci Rep. 2018 Sep 28;38(5):BSR20180566. doi: 10.1042/BSR20180566. Print 2018 Oct 31. Biosci Rep. 2018. PMID: 29884766 Free PMC article.
-
Long non-coding RNA TUG1 promotes cell proliferation and metastasis by negatively regulating miR-300 in gallbladder carcinoma.Biomed Pharmacother. 2017 Apr;88:863-869. doi: 10.1016/j.biopha.2017.01.150. Epub 2017 Feb 6. Biomed Pharmacother. 2017. PMID: 28178615
-
Long non-coding TUG1 accelerates prostate cancer progression through regulating miR-128-3p/YES1 axis.Eur Rev Med Pharmacol Sci. 2020 Jan;24(2):619-632. doi: 10.26355/eurrev_202001_20038. Eur Rev Med Pharmacol Sci. 2020. PMID: 32016963
-
Taurine-upregulated gene 1 contributes to cancers through sponging microRNA.Acta Biochim Biophys Sin (Shanghai). 2019 Feb 1;51(2):123-130. doi: 10.1093/abbs/gmy156. Acta Biochim Biophys Sin (Shanghai). 2019. PMID: 30590378 Review.
-
Pathophysiological Functions of the lncRNA TUG1.Curr Pharm Des. 2020;26(6):688-700. doi: 10.2174/1381612826666191227154009. Curr Pharm Des. 2020. PMID: 31880241 Review.
Cited by
-
Role of long noncoding RNA taurine-upregulated gene 1 in cancers.Mol Med. 2021 May 26;27(1):51. doi: 10.1186/s10020-021-00312-4. Mol Med. 2021. PMID: 34039257 Free PMC article. Review.
-
Pan-cancer characterization of long non-coding RNA and DNA methylation mediated transcriptional dysregulation.EBioMedicine. 2021 Jun;68:103399. doi: 10.1016/j.ebiom.2021.103399. Epub 2021 May 24. EBioMedicine. 2021. PMID: 34044218 Free PMC article.
-
miR-26a is a Key Therapeutic Target with Enormous Potential in the Diagnosis and Prognosis of Human Disease.Curr Med Chem. 2024;31(18):2550-2570. doi: 10.2174/0109298673271808231116075056. Curr Med Chem. 2024. PMID: 38204224 Review.
-
lncRNA TUG1 modulates proliferation, apoptosis, invasion, and angiogenesis via targeting miR-29b in trophoblast cells.Hum Genomics. 2019 Sep 13;13(1):50. doi: 10.1186/s40246-019-0237-z. Hum Genomics. 2019. Retraction in: Hum Genomics. 2022 May 18;16(1):17. doi: 10.1186/s40246-022-00389-w. PMID: 31519209 Free PMC article. Retracted.
-
Prognostic Significance of Amino Acid Metabolism-Related Genes in Prostate Cancer Retrieved by Machine Learning.Cancers (Basel). 2023 Feb 18;15(4):1309. doi: 10.3390/cancers15041309. Cancers (Basel). 2023. PMID: 36831650 Free PMC article.
References
-
- Srigley J.R., Amin M., Boccon-Gibod L., Egevad L., Epstein J.I., Humphrey P.A. et al. (2005) Prognostic and predictive factors in prostate cancer: historical perspectives and recent international consensus initiatives. Scand. J. Urol. Nephrol. Suppl. 216, 8–19 10.1080/03008880510030914 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

