Recombinant MDA-7/IL24 Suppresses Prostate Cancer Bone Metastasis through Downregulation of the Akt/Mcl-1 Pathway
- PMID: 29934341
- PMCID: PMC7598934
- DOI: 10.1158/1535-7163.MCT-17-1002
Recombinant MDA-7/IL24 Suppresses Prostate Cancer Bone Metastasis through Downregulation of the Akt/Mcl-1 Pathway
Abstract
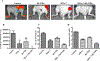
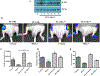
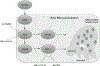
Prostate cancer is a principal cause of cancer-associated morbidity in men. Although 5-year survival of patients with localized prostate cancer approaches 100%, survival decreases precipitously after metastasis. Bone is the preferred site for disseminated prostate cancer cell colonization, altering the equilibrium of bone homeostasis resulting in weak and fragile bones. Currently, no curative options are available for prostate cancer bone metastasis. Melanoma differentiation associated gene-7 (MDA-7)/IL24 is a well-studied cytokine established as a therapeutic in a wide array of cancers upon delivery as a gene therapy. In this study, we explored the potential anticancer properties of MDA-7/IL24 delivered as a recombinant protein. Using bone metastasis experimental models, animals treated with recombinant MDA-7/IL24 had significantly less metastatic lesions in their femurs as compared with controls. The inhibitory effects of MDA-7/IL24 on bone metastasis resulted from prostate cancer-selective killing and inhibition of osteoclast differentiation, which is necessary for bone resorption. Gain- and loss-of-function genetic approaches document that prosurvival Akt and Mcl-1 pathways are critically important in the antibone metastatic activity of MDA-7/IL24. Our previous findings showed that MDA-7/IL24 gene therapy plus Mcl-1 inhibitors cooperate synergistically. Similarly, an Mcl-1 small-molecule inhibitor synergized with MDA-7/IL24 and induced robust antibone metastatic activity. These results expand the potential applications of MDA-7/IL24 as an anticancer molecule and demonstrate that purified recombinant protein is nontoxic in preclinical animal models and has profound inhibitory effects on bone metastasis, which can be enhanced further when combined with an Mcl-1 inhibitory small molecule. Mol Cancer Ther; 17(9); 1951-60. ©2018 AACR.
©2018 American Association for Cancer Research.
Figures



Similar articles
-
Engineering T Cells to Express Tumoricidal MDA-7/IL24 Enhances Cancer Immunotherapy.Cancer Res. 2021 May 1;81(9):2429-2441. doi: 10.1158/0008-5472.CAN-20-2604. Epub 2021 Mar 16. Cancer Res. 2021. PMID: 33727225 Free PMC article.
-
Apogossypol derivative BI-97C1 (Sabutoclax) targeting Mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity.Proc Natl Acad Sci U S A. 2011 May 24;108(21):8785-90. doi: 10.1073/pnas.1100769108. Epub 2011 May 9. Proc Natl Acad Sci U S A. 2011. PMID: 21555592 Free PMC article.
-
Mechanism by which Mcl-1 regulates cancer-specific apoptosis triggered by mda-7/IL-24, an IL-10-related cytokine.Cancer Res. 2010 Jun 15;70(12):5034-45. doi: 10.1158/0008-5472.CAN-10-0563. Epub 2010 May 25. Cancer Res. 2010. PMID: 20501829 Free PMC article.
-
Ras and Wnt Interaction Contribute in Prostate Cancer Bone Metastasis.Molecules. 2020 May 20;25(10):2380. doi: 10.3390/molecules25102380. Molecules. 2020. PMID: 32443915 Free PMC article. Review.
-
MDA-7/IL-24: multifunctional cancer killing cytokine.Adv Exp Med Biol. 2014;818:127-53. doi: 10.1007/978-1-4471-6458-6_6. Adv Exp Med Biol. 2014. PMID: 25001534 Free PMC article. Review.
Cited by
-
Precision Targets for Intercepting the Lethal Progression of Prostate Cancer: Potential Avenues for Personalized Therapy.Cancers (Basel). 2022 Feb 11;14(4):892. doi: 10.3390/cancers14040892. Cancers (Basel). 2022. PMID: 35205640 Free PMC article. Review.
-
PIKFYVE inhibitors trigger interleukin-24-dependent cell death of autophagy-dependent melanoma.Mol Oncol. 2024 Apr;18(4):988-1011. doi: 10.1002/1878-0261.13607. Epub 2024 Feb 27. Mol Oncol. 2024. PMID: 38414326 Free PMC article.
-
Deguelin suppresses non-small cell lung cancer by inhibiting EGFR signaling and promoting GSK3β/FBW7-mediated Mcl-1 destabilization.Cell Death Dis. 2020 Feb 21;11(2):143. doi: 10.1038/s41419-020-2344-0. Cell Death Dis. 2020. PMID: 32081857 Free PMC article.
-
MDA-9/Syntenin in the tumor and microenvironment defines prostate cancer bone metastasis.Proc Natl Acad Sci U S A. 2023 Nov 7;120(45):e2307094120. doi: 10.1073/pnas.2307094120. Epub 2023 Nov 3. Proc Natl Acad Sci U S A. 2023. PMID: 37922327 Free PMC article.
-
AKT in Bone Metastasis of Solid Tumors: A Comprehensive Review.Cancers (Basel). 2021 May 11;13(10):2287. doi: 10.3390/cancers13102287. Cancers (Basel). 2021. PMID: 34064589 Free PMC article. Review.
References
-
- Rigaud J, Tiguert R, Le Normand L, Karam G, Glemain P, Buzelin JM, et al. Prognostic value of bone scan in patients with metastatic prostate cancer treated initially with androgen deprivation therapy. J Urol 2002;168:1423–6. - PubMed
-
- Ye L, Kynaston HG, Jiang WG. Bone metastasis in prostate cancer: molecular and cellular mechanisms (Review). Int J Mol Med 2007;20:103–11. - PubMed
-
- Coghlin C, Murray GI. Current and emerging concepts in tumour metastasis. J Pathol 2010;222:1–15. - PubMed
-
- Tanaka Y, Nakayamada S, Okada Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Current drug targets Inflammation and allergy 2005;4:325–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

