Mitochondrial Chaperones in the Brain: Safeguarding Brain Health and Metabolism?
- PMID: 29755410
- PMCID: PMC5932182
- DOI: 10.3389/fendo.2018.00196
Mitochondrial Chaperones in the Brain: Safeguarding Brain Health and Metabolism?
Abstract
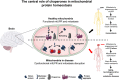
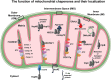
The brain orchestrates organ function and regulates whole body metabolism by the concerted action of neurons and glia cells in the central nervous system. To do so, the brain has tremendously high energy consumption and relies mainly on glucose utilization and mitochondrial function in order to exert its function. As a consequence of high rate metabolism, mitochondria in the brain accumulate errors over time, such as mitochondrial DNA (mtDNA) mutations, reactive oxygen species, and misfolded and aggregated proteins. Thus, mitochondria need to employ specific mechanisms to avoid or ameliorate the rise of damaged proteins that contribute to aberrant mitochondrial function and oxidative stress. To maintain mitochondria homeostasis (mitostasis), cells evolved molecular chaperones that shuttle, refold, or in coordination with proteolytic systems, help to maintain a low steady-state level of misfolded/aggregated proteins. Their importance is exemplified by the occurrence of various brain diseases which exhibit reduced action of chaperones. Chaperone loss (expression and/or function) has been observed during aging, metabolic diseases such as type 2 diabetes and in neurodegenerative diseases such as Alzheimer's (AD), Parkinson's (PD) or even Huntington's (HD) diseases, where the accumulation of damage proteins is evidenced. Within this perspective, we propose that proper brain function is maintained by the joint action of mitochondrial chaperones to ensure and maintain mitostasis contributing to brain health, and that upon failure, alter brain function which can cause metabolic diseases.
Keywords: brain; chaperones; insulin signaling; mitochondria homeostasis; mitochondrial dysfunction; neurodegeneration.
Figures
Similar articles
-
Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders.J Neurol Sci. 2007 Jun 15;257(1-2):221-39. doi: 10.1016/j.jns.2007.01.033. Epub 2007 Apr 25. J Neurol Sci. 2007. PMID: 17462670 Review.
-
Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich's ataxia.J Neurol Sci. 2005 Jun 15;233(1-2):145-62. doi: 10.1016/j.jns.2005.03.012. J Neurol Sci. 2005. PMID: 15896810 Review.
-
Mitochondria, oxidative stress and neurodegeneration.J Neurol Sci. 2012 Nov 15;322(1-2):254-62. doi: 10.1016/j.jns.2012.05.030. Epub 2012 Jun 4. J Neurol Sci. 2012. PMID: 22669122 Review.
-
Linking mitochondrial dysfunction, metabolic syndrome and stress signaling in Neurodegeneration.Biochim Biophys Acta Mol Basis Dis. 2017 May;1863(5):1132-1146. doi: 10.1016/j.bbadis.2016.06.015. Epub 2016 Jun 21. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 27345267 Review.
-
Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders.Neurol Res. 2017 Jan;39(1):73-82. doi: 10.1080/01616412.2016.1251711. Epub 2016 Nov 3. Neurol Res. 2017. PMID: 27809706 Review.
Cited by
-
Modeling alcohol-induced neurotoxicity using human induced pluripotent stem cell-derived three-dimensional cerebral organoids.Transl Psychiatry. 2020 Oct 13;10(1):347. doi: 10.1038/s41398-020-01029-4. Transl Psychiatry. 2020. PMID: 33051447 Free PMC article.
-
Climate Stressors and Physiological Dysregulations: Mechanistic Connections to Pathologies.Int J Environ Res Public Health. 2023 Dec 23;21(1):28. doi: 10.3390/ijerph21010028. Int J Environ Res Public Health. 2023. PMID: 38248493 Free PMC article. Review.
-
DNAJA3/Tid1 Is Required for Mitochondrial DNA Maintenance and Regulates Migration and Invasion of Human Gastric Cancer Cells.Cancers (Basel). 2020 Nov 20;12(11):3463. doi: 10.3390/cancers12113463. Cancers (Basel). 2020. PMID: 33233689 Free PMC article.
-
Downregulation of mitochondrial metabolism is a driver for fast skeletal muscle loss during mouse aging.Commun Biol. 2023 Dec 8;6(1):1240. doi: 10.1038/s42003-023-05595-3. Commun Biol. 2023. PMID: 38066057 Free PMC article.
-
Chaperones-A New Class of Potential Therapeutic Targets in Alzheimer's Disease.Int J Mol Sci. 2024 Mar 17;25(6):3401. doi: 10.3390/ijms25063401. Int J Mol Sci. 2024. PMID: 38542375 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

