Glucosamine-Induced Autophagy through AMPK⁻mTOR Pathway Attenuates Lipofuscin-Like Autofluorescence in Human Retinal Pigment Epithelial Cells In Vitro
- PMID: 29747425
- PMCID: PMC5983587
- DOI: 10.3390/ijms19051416
Glucosamine-Induced Autophagy through AMPK⁻mTOR Pathway Attenuates Lipofuscin-Like Autofluorescence in Human Retinal Pigment Epithelial Cells In Vitro
Abstract
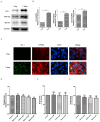
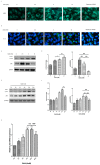
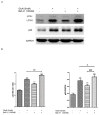
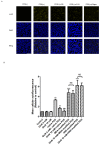
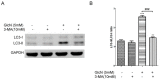
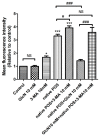
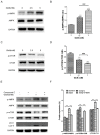
Age-related macular degeneration (AMD) is a vision-threatening age-associated disease. The retinal pigment epithelial (RPE) cells phagocytose and digest photoreceptor outer segment (POS). Incomplete digestion of POS leads to lipofuscin accumulation, which contributes to the pathology of the AMD. Autophagy could help reduce the amount of lipofuscin accumulation. In the present study, we evaluated the effects of glucosamine (GlcN), a natural supplement, on the induction of autophagy and POS-derived lipofuscin-like autofluorescence (LLAF) in ARPE-19 cells in vitro, and investigated the potential molecular pathway involved. Our results revealed that GlcN had no effect on phagocytosis of POS at the lower doses. GlcN treatment induced autophagy in cells. GlcN decreased the LLAF in native POS-treated cells, whereas malondialdehyde or 4-hydroxynonenal-modified POS attenuated this effect. 3-Methyladenine inhibited GlcN-induced autophagy and attenuated the effect of GlcN on the decrease of the native POS-derived LLAF. Furthermore, GlcN induced the phosphorylation of AMP-activated protein kinase (AMPK) and inhibited the phosphorylation of mammalian target of rapamycin (mTOR), whereas Compound C inhibited these effects of GlcN. Altogether, these results suggest that GlcN decreased the native POS-derived LLAF through induction of autophagy, at least in part, by the AMPK⁻mTOR pathway. This mechanism has potential for the preventive treatment of lipofuscin-related retinal degeneration such as AMD.
Keywords: autophagy; glucosamine; lipofuscin; retinal pigment epithelial cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells.Exp Eye Res. 2010 Mar;90(3):465-71. doi: 10.1016/j.exer.2009.12.011. Epub 2010 Jan 6. Exp Eye Res. 2010. PMID: 20059996
-
Inhibition or Stimulation of Autophagy Affects Early Formation of Lipofuscin-Like Autofluorescence in the Retinal Pigment Epithelium Cell.Int J Mol Sci. 2017 Mar 29;18(4):728. doi: 10.3390/ijms18040728. Int J Mol Sci. 2017. PMID: 28353645 Free PMC article.
-
Complement and UV-irradiated photoreceptor outer segments increase the cytokine secretion by retinal pigment epithelial cells.Invest Ophthalmol Vis Sci. 2012 Mar 15;53(3):1406-13. doi: 10.1167/iovs.11-8889. Invest Ophthalmol Vis Sci. 2012. PMID: 22323489
-
5'-Adenosine monophosphate-activated protein kinase--mammalian target of rapamycin axis as therapeutic target for age-related macular degeneration.Rejuvenation Res. 2011 Dec;14(6):651-60. doi: 10.1089/rej.2011.1220. Epub 2011 Oct 18. Rejuvenation Res. 2011. PMID: 22007913 Review.
-
Rescue of compromised lysosomes enhances degradation of photoreceptor outer segments and reduces lipofuscin-like autofluorescence in retinal pigmented epithelial cells.Adv Exp Med Biol. 2014;801:105-11. doi: 10.1007/978-1-4614-3209-8_14. Adv Exp Med Biol. 2014. PMID: 24664687 Free PMC article. Review.
Cited by
-
Autophagy in Age-Related Macular Degeneration: A Regulatory Mechanism of Oxidative Stress.Oxid Med Cell Longev. 2020 Aug 8;2020:2896036. doi: 10.1155/2020/2896036. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32831993 Free PMC article. Review.
-
The association between age-related macular degeneration and risk of Parkinson disease: A systematic review and meta-analysis.Medicine (Baltimore). 2024 Nov 15;103(46):e40524. doi: 10.1097/MD.0000000000040524. Medicine (Baltimore). 2024. PMID: 39560583 Free PMC article.
-
Identification and Validation of Autophagy-Related Genes in Diabetic Retinopathy.Front Endocrinol (Lausanne). 2022 Apr 29;13:867600. doi: 10.3389/fendo.2022.867600. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35574010 Free PMC article.
-
Autophagy in the retinal pigment epithelium: a new vision and future challenges.FEBS J. 2022 Nov;289(22):7199-7212. doi: 10.1111/febs.16018. Epub 2021 May 31. FEBS J. 2022. PMID: 33993621 Free PMC article. Review.
-
mTOR Signalling Pathway: A Potential Therapeutic Target for Ocular Neurodegenerative Diseases.Antioxidants (Basel). 2022 Jun 29;11(7):1304. doi: 10.3390/antiox11071304. Antioxidants (Basel). 2022. PMID: 35883796 Free PMC article. Review.
References
-
- Bird A.C., Bressler N.M., Bressler S.B., Chisholm I.H., Coscas G., Davis M.D., de Jong P.T., Klaver C.C., Klein B.E., Klein R., et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv. Ophthalmol. 1995;39:367–374. doi: 10.1016/S0039-6257(05)80092-X. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

